Shale on:
[Wikipedia]
[Google]
[Amazon]
Shale is a fine-grained, clastic
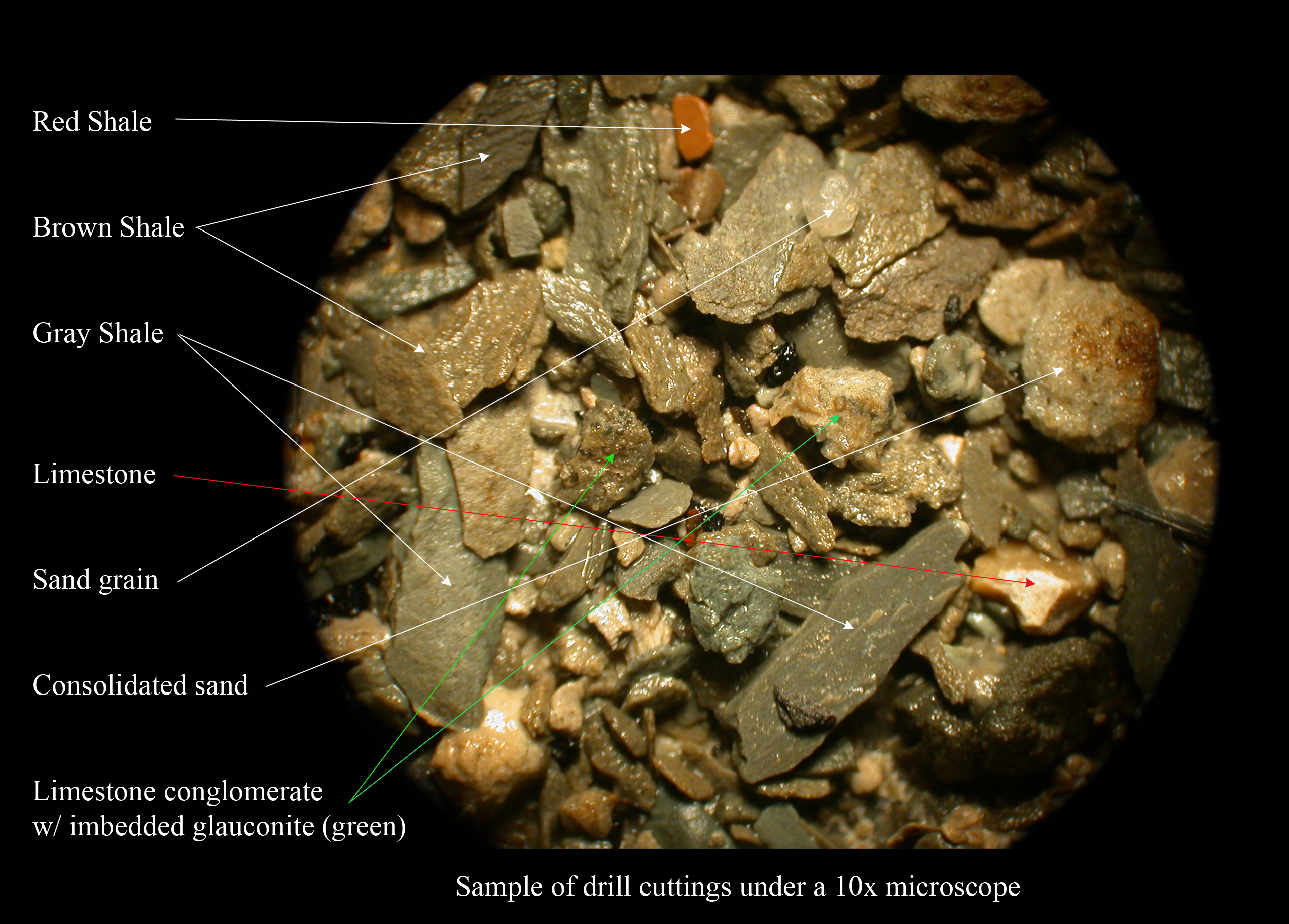
 Shales are typically gray in color and are composed of clay minerals and quartz grains. The addition of variable amounts of minor constituents alters the color of the rock. Red, brown and green colors are indicative of
Shales are typically gray in color and are composed of clay minerals and quartz grains. The addition of variable amounts of minor constituents alters the color of the rock. Red, brown and green colors are indicative of
File:Shale in Potokgraben.jpg, Shale in Potokgraben, the
sedimentary rock
Sedimentary rocks are types of rock that are formed by the accumulation or deposition of mineral or organic particles at Earth's surface, followed by cementation. Sedimentation is the collective name for processes that cause these particles ...
formed from mud that is a mix of flakes of clay minerals
Clay minerals are hydrous aluminium phyllosilicates (e.g. kaolin, Al2 Si2 O5( OH)4), sometimes with variable amounts of iron, magnesium, alkali metals, alkaline earths, and other cations found on or near some planetary surfaces.
Clay mineral ...
(hydrous aluminium phyllosilicates, e.g. kaolin, Al2 Si2 O5( OH)4) and tiny fragments (silt
Silt is granular material of a size between sand and clay and composed mostly of broken grains of quartz. Silt may occur as a soil (often mixed with sand or clay) or as sediment mixed in suspension with water. Silt usually has a floury feel when ...
-sized particles) of other minerals, especially quartz
Quartz is a hard, crystalline mineral composed of silica (silicon dioxide). The atoms are linked in a continuous framework of SiO4 silicon-oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall chemical form ...
and calcite
Calcite is a Carbonate minerals, carbonate mineral and the most stable Polymorphism (materials science), polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on ...
.Blatt, Harvey and Robert J. Tracy (1996) ''Petrology: Igneous, Sedimentary and Metamorphic'', 2nd ed., Freeman, pp. 281–292 Shale is characterized by its tendency to split into thin layers ( laminae) less than one centimeter in thickness. This property is called '' fissility''. Shale is the most common sedimentary rock.
The term ''shale'' is sometimes applied more broadly, as essentially a synonym for mudrock
Mudrocks are a class of fine-grained siliciclastic sedimentary rocks. The varying types of mudrocks include siltstone, claystone, mudstone, slate, and shale. Most of the particles of which the stone is composed are less than and are too ...
, rather than in the more narrow sense of clay-rich fissile mudrock.
Texture
Shale typically exhibits varying degrees of fissility. Because of the parallel orientation of clay mineral flakes in shale, it breaks into thin layers, often splintery and usually parallel to the otherwise indistinguishable bedding planes.Blatt, Harvey and Robert J. Tracy (1996) ''Petrology: Igneous, Sedimentary and Metamorphic'', 2nd ed., Freeman, pp. 281–292 Non-fissilerocks
In geology, rock (or stone) is any naturally occurring solid mass or aggregate of minerals or mineraloid matter. It is categorized by the minerals included, its chemical composition, and the way in which it is formed. Rocks form the Earth's ...
of similar composition and particle size (less than 0.0625 mm) are described as mudstone
Mudstone, a type of mudrock, is a fine-grained sedimentary rock whose original constituents were clays or muds. Mudstone is distinguished from '' shale'' by its lack of fissility (parallel layering).Blatt, H., and R.J. Tracy, 1996, ''Petrology. ...
s (1/3 to 2/3 silt particles) or claystones
Mudrocks are a class of fine-grained siliciclastic sedimentary rocks. The varying types of mudrocks include siltstone, claystone, mudstone, slate, and shale. Most of the particles of which the stone is composed are less than and are too smal ...
(less than 1/3 silt). Rocks with similar particle sizes but with less clay (greater than 2/3 silt) and therefore grittier are siltstones.

Composition and color
 Shales are typically gray in color and are composed of clay minerals and quartz grains. The addition of variable amounts of minor constituents alters the color of the rock. Red, brown and green colors are indicative of
Shales are typically gray in color and are composed of clay minerals and quartz grains. The addition of variable amounts of minor constituents alters the color of the rock. Red, brown and green colors are indicative of ferric oxide
Iron(III) oxide or ferric oxide is the inorganic compound with the formula Fe2O3. It is one of the three main oxides of iron, the other two being iron(II) oxide (FeO), which is rare; and iron(II,III) oxide (Fe3O4), which also occurs naturall ...
(hematite
Hematite (), also spelled as haematite, is a common iron oxide compound with the formula, Fe2O3 and is widely found in rocks and soils. Hematite crystals belong to the rhombohedral lattice system which is designated the alpha polymorph of . ...
– reds), iron hydroxide
Iron oxides are chemical compounds composed of iron and oxygen. Several iron oxides are recognized. All are black magnetic solids. Often they are non-stoichiometric. Oxyhydroxides are a related class of compounds, perhaps the best known of whi ...
(goethite
Goethite (, ) is a mineral of the diaspore group, consisting of iron(III) oxide-hydroxide, specifically the "α" polymorph. It is found in soil and other low-temperature environments such as sediment. Goethite has been well known since ancient t ...
– browns and limonite – yellow), or micaceous
Micas ( ) are a group of silicate minerals whose outstanding physical characteristic is that individual mica crystals can easily be split into extremely thin elastic plates. This characteristic is described as perfect basal cleavage. Mica is ...
minerals ( chlorite, biotite and illite
Illite is a group of closely related non-expanding clay minerals. Illite is a secondary mineral precipitate, and an example of a phyllosilicate, or layered alumino-silicate. Its structure is a 2:1 sandwich of silica tetrahedron (T) – alumina ...
– greens). The color shifts from reddish to greenish as iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in f ...
in the oxidized
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
(ferric
In chemistry, iron(III) refers to the element iron in its +3 oxidation state. In ionic compounds (salts), such an atom may occur as a separate cation (positive ion) denoted by Fe3+.
The adjective ferric or the prefix ferri- is often used to sp ...
) state is converted to iron in the reduced (ferrous
In chemistry, the adjective Ferrous indicates a compound that contains iron(II), meaning iron in its +2 oxidation state, possibly as the divalent cation Fe2+. It is opposed to "ferric" or iron(III), meaning iron in its +3 oxidation state, such a ...
) state. Black shale results from the presence of greater than one percent carbonaceous
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon makes up ...
material and indicates a reducing environment. Pale blue to blue-green shales typically are rich in carbonate minerals
Carbonate minerals are those minerals containing the carbonate ion, .
Carbonate divisions Anhydrous carbonates
*Calcite group: trigonal
**Calcite CaCO3
**Gaspéite (Ni,Mg,Fe2+)CO3
**Magnesite MgCO3
**Otavite CdCO3
**Rhodochrosite MnCO3
**Sider ...
.
Clays are the major constituent of shales and other mudrocks. The clay minerals represented are largely kaolinite, montmorillonite
Montmorillonite is a very soft phyllosilicate group of minerals that form when they precipitate from water solution as microscopic crystals, known as clay. It is named after Montmorillon in France. Montmorillonite, a member of the smectite gro ...
and illite. Clay minerals of Late Tertiary
Tertiary ( ) is a widely used but obsolete term for the geologic period from 66 million to 2.6 million years ago.
The period began with the demise of the non-avian dinosaurs in the Cretaceous–Paleogene extinction event, at the start ...
mudstones are expandable smectites, whereas in older rocks (especially in mid-to early Paleozoic
The Paleozoic (or Palaeozoic) Era is the earliest of three geologic eras of the Phanerozoic Eon.
The name ''Paleozoic'' ( ;) was coined by the British geologist Adam Sedgwick in 1838
by combining the Greek words ''palaiós'' (, "old") and ' ...
shales) illites predominate. The transformation of smectite to illite produces silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is ...
, sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable iso ...
, calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to ...
, magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ta ...
, iron and water. These released elements form authigenic
Authigenesis is the process whereby a mineral or sedimentary rock deposit is generated where it is found or observed. Such deposits are described as authigenic. Authigenic sedimentary minerals form during sedimentation by precipitation or recrys ...
quartz
Quartz is a hard, crystalline mineral composed of silica (silicon dioxide). The atoms are linked in a continuous framework of SiO4 silicon-oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall chemical form ...
, chert
Chert () is a hard, fine-grained sedimentary rock composed of microcrystalline or cryptocrystalline quartz, the mineral form of silicon dioxide (SiO2). Chert is characteristically of biological origin, but may also occur inorganically as a prec ...
, calcite
Calcite is a Carbonate minerals, carbonate mineral and the most stable Polymorphism (materials science), polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on ...
, dolomite Dolomite may refer to:
*Dolomite (mineral), a carbonate mineral
*Dolomite (rock), also known as dolostone, a sedimentary carbonate rock
*Dolomite, Alabama, United States, an unincorporated community
*Dolomite, California, United States, an unincor ...
, ankerite
Ankerite is a calcium, iron, magnesium, manganese carbonate mineral of the group of rhombohedral carbonates with the chemical formula . In composition it is closely related to dolomite, but differs from this in having magnesium replaced by varyin ...
, hematite and albite
Albite is a plagioclase feldspar mineral. It is the sodium endmember of the plagioclase solid solution series. It represents a plagioclase with less than 10% anorthite content. The pure albite endmember has the formula . It is a tectosilicate. I ...
, all trace to minor (except quartz) minerals found in shales and other mudrocks. A typical shale is composed of about 58% clay minerals, 28% quartz, 6% feldspar
Feldspars are a group of rock-forming aluminium tectosilicate minerals, also containing other cations such as sodium, calcium, potassium, or barium. The most common members of the feldspar group are the ''plagioclase'' (sodium-calcium) feldsp ...
, 5% carbonate minerals, and 2% iron oxides
Iron oxides are chemical compounds composed of iron and oxygen. Several iron oxides are recognized. All are black magnetic solids. Often they are non-stoichiometric. Oxyhydroxides are a related class of compounds, perhaps the best known of which ...
. Most of the quartz is detrital
Detritus (; adj. ''detrital'' ) is particles of rock derived from pre-existing rock through weathering and erosion.Essentials of Geology, 3rd Ed, Stephen Marshak, p G-7 A fragment of detritus is called a clast.Essentials of Geology, 3rd Ed, Stephen ...
(part of the original sediments that formed the shale) rather than authigenic (crystallized within the shale after deposition).
Shales and other mudrocks contain roughly 95 percent of the organic matter in all sedimentary rocks. However, this amounts to less than one percent by mass in an average shale. Black shales, which form in anoxic
The term anoxia means a total depletion in the level of oxygen, an extreme form of hypoxia or "low oxygen". The terms anoxia and hypoxia are used in various contexts:
* Anoxic waters, sea water, fresh water or groundwater that are depleted of diss ...
conditions, contain reduced free carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
along with ferrous iron
In chemistry, iron(II) refers to the element iron in its +2 oxidation state. In ionic compounds (salts), such an atom may occur as a separate cation (positive ion) denoted by Fe2+.
The adjective ferrous or the prefix ferro- is often used to ...
(Fe2+) and sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
(S2−). Amorphous
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid, glassy solid) is a solid that lacks the long-range order that is characteristic of a crystal.
Etymology
The term comes from the Greek ''a'' ("wi ...
iron sulfide
Iron sulfide or Iron sulphide can refer to range of chemical compounds composed of iron and sulfur.
Minerals
By increasing order of stability:
* Iron(II) sulfide, FeS
* Greigite, Fe3S4 (cubic)
* Pyrrhotite, Fe1−xS (where x = 0 to 0.2) (monocli ...
, along with carbon, produce the black coloration. Because amorphous iron sulfide gradually converts to pyrite
The mineral pyrite (), or iron pyrite, also known as fool's gold, is an iron sulfide with the chemical formula Iron, FeSulfur, S2 (iron (II) disulfide). Pyrite is the most abundant sulfide mineral.
Pyrite's metallic Luster (mineralogy), lust ...
, which is not an important pigment, young shales may be quite dark from their iron sulfide content, in spite of a modest carbon content (less than 1%), while a black color in an ancient shale indicates a high carbon content.
Most shales are marine in origin, and the groundwater
Groundwater is the water present beneath Earth's surface in rock and soil pore spaces and in the fractures of rock formations. About 30 percent of all readily available freshwater in the world is groundwater. A unit of rock or an unconsolidate ...
in shale formations is often highly saline. There is evidence that shale acts as a semipermeable medium, allowing water to pass through while retaining dissolved salts.
Formation
The fine particles that compose shale can remain suspended in water long after the larger particles of sand have been deposited. As a result, shales are typically deposited in very slow moving water and are often found in lakes andlagoon
A lagoon is a shallow body of water separated from a larger body of water by a narrow landform, such as reefs, barrier islands, barrier peninsulas, or isthmuses. Lagoons are commonly divided into ''coastal lagoons'' (or ''barrier lagoons'') a ...
al deposits, in river delta
A river delta is a landform shaped like a triangle, created by deposition (geology), deposition of sediment that is carried by a river and enters slower-moving or stagnant water. This occurs where a river enters an ocean, sea, estuary, lake, res ...
s, on floodplain
A floodplain or flood plain or bottomlands is an area of land adjacent to a river which stretches from the banks of its channel to the base of the enclosing valley walls, and which experiences flooding during periods of high discharge.Goudi ...
s and offshore below the wave base
The wave base, in physical oceanography, is the maximum depth at which a water wave's passage causes significant water motion. At water depths deeper than the wave base, bottom sediments and the seafloor are no longer stirred by the wave motion a ...
. Thick deposits of shale are found near ancient continental margins and foreland basins
Foreland may refer to:
* a landform projecting into the sea, such as a headland or a promontory
* an area of land in front of something
**Foreland basin, in geology, the zone that receives sediment from an adjacent mountain chain
**Glacier foreland ...
. Some of the most widespread shale formations were deposited by epicontinental sea
An inland sea (also known as an epeiric sea or an epicontinental sea) is a continental body of water which is very large and is either completely surrounded by dry land or connected to an ocean by a river, strait, or "arm of the sea". An inland s ...
s. Black shales are common in Cretaceous
The Cretaceous ( ) is a geological period that lasted from about 145 to 66 million years ago (Mya). It is the third and final period of the Mesozoic Era, as well as the longest. At around 79 million years, it is the longest geological period of th ...
strata on the margins of the Atlantic Ocean
The Atlantic Ocean is the second-largest of the world's five oceans, with an area of about . It covers approximately 20% of Earth's surface and about 29% of its water surface area. It is known to separate the " Old World" of Africa, Europe ...
, where they were deposited in fault-bounded silled basins associated with the opening of the Atlantic during the breakup of Pangea
Pangaea or Pangea () was a supercontinent that existed during the late Paleozoic and early Mesozoic eras. It assembled from the earlier continental units of Gondwana, Euramerica and Siberia during the Carboniferous approximately 335 million y ...
. These basins were anoxic, in part because of restricted circulation in the narrow Atlantic, and in part because the very warm Cretaceous seas lacked the circulation of cold bottom water that oxygenates the deep oceans today.
Most clay must be deposited as aggregates and floccules, since the settling rate of individual clay particles is extremely slow. Flocculation
Flocculation, in the field of chemistry, is a process by which colloidal particles come out of suspension to sediment under the form of floc or flake, either spontaneously or due to the addition of a clarifying agent. The action differs from pr ...
is very rapid once the clay encounters highly saline sea water. Whereas individual clay particles are less than 4 microns in size, the clumps of clay particles produced by flocculation vary in size from a few tens of microns to over 700 microns in diameter. The floccules start out water-rich, but much of the water is expelled from the floccules as the clay minerals bind more tightly together over time (a process called syneresis). Clay pelletization by organisms that filter feed
Filter feeders are a sub-group of suspension feeding animals that feed by straining suspended matter and food particles from water, typically by passing the water over a specialized filtering structure. Some animals that use this method of feedin ...
is important where flocculation is inhibited. Filter feeders produce an estimated 12 metric tons of clay pellets per square kilometer per year along the U.S. Gulf Coast.
As sediments continue to accumulate, the older, more deeply buried sediments begin to undergo diagenesis
Diagenesis () is the process that describes physical and chemical changes in sediments first caused by water-rock interactions, microbial activity, and compaction after their deposition. Increased pressure and temperature only start to play a ...
. This mostly consists of compaction and lithification
Lithification (from the Ancient Greek word ''lithos'' meaning 'rock' and the Latin-derived suffix ''-ific'') is the process in which sediments compact under pressure, expel connate fluids, and gradually become solid rock. Essentially, lithificati ...
of the clay and silt particles. Early stages of diagenesis, described as ''eogenesis'', take place at shallow depths (a few tens of meters) and are characterized by bioturbation
Bioturbation is defined as the reworking of soils and sediments by animals or plants. It includes burrowing, ingestion, and defecation of sediment grains. Bioturbating activities have a profound effect on the environment and are thought to be a pr ...
and mineralogical changes in the sediments, with only slight compaction. Pyrite
The mineral pyrite (), or iron pyrite, also known as fool's gold, is an iron sulfide with the chemical formula Iron, FeSulfur, S2 (iron (II) disulfide). Pyrite is the most abundant sulfide mineral.
Pyrite's metallic Luster (mineralogy), lust ...
may be formed in anoxic mud at this stage of diagenesis.
Deeper burial is accompanied by ''mesogenesis'', during which most of the compaction and lithification takes place. As the sediments come under increasing pressure from overlying sediments, sediment grains move into more compact arrangements, ductile grains (such as clay mineral grains) are deformed, and pore space
Porosity or void fraction is a measure of the void (i.e. "empty") spaces in a material, and is a fraction of the volume of voids over the total volume, between 0 and 1, or as a percentage between 0% and 100%. Strictly speaking, some tests measure ...
is reduced. In addition to this physical compaction, chemical compaction may take place via pressure solution
In structural geology and diagenesis, pressure solution or pressure dissolution is a deformation mechanism that involves the dissolution of minerals at grain-to-grain contacts into an aqueous pore fluid in areas of relatively high stress and e ...
. Points of contact between grains are under the greatest strain, and the strained mineral is more soluble
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubil ...
than the rest of the grain. As a result, the contact points are dissolved away, allowing the grains to come into closer contact.
It is during compaction that shale develops its fissility, likely through mechanical compaction of the original open framework of clay particles. The particles become strongly oriented into parallel layers that give the shale its distinctive fabric. Fissility likely develops early in the compaction process, at relatively shallow depth, since fissility does not seem to vary with depth in thick formations. Kaolinite flakes have less tendency to align in parallel layers than other clays, so kaolinite-rich clay is more likely to form nonfissile mudstone than shale. On the other hand, black shales often have very pronounced fissility (''paper shales'') due to binding of hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ex ...
molecules to the faces of the clay particles, which weakens the binding between particles.
Lithification follows closely on compaction, as increased temperatures at depth hasten deposition of cement
A cement is a binder, a chemical substance used for construction that sets, hardens, and adheres to other materials to bind them together. Cement is seldom used on its own, but rather to bind sand and gravel ( aggregate) together. Cement mix ...
that binds the grains together. Pressure solution contributes to cementing, as the mineral dissolved from strained contact points is redeposited in the unstrained pore spaces. The clay minerals may be altered as well. For example, smectite
A smectite (from ancient Greek ''σμηκτός'' smektos 'lubricated'; ''σμηκτρίς'' smektris 'walker's earth', 'fuller's earth'; rubbing earth; earth that has the property of cleaning) is a mineral mixtures of various swelling sheet sil ...
is altered to illite
Illite is a group of closely related non-expanding clay minerals. Illite is a secondary mineral precipitate, and an example of a phyllosilicate, or layered alumino-silicate. Its structure is a 2:1 sandwich of silica tetrahedron (T) – alumina ...
at temperatures of about , releasing water in the process. Other alteration reactions include the alteration of smectite to chlorite
The chlorite ion, or chlorine dioxide anion, is the halite with the chemical formula of . A chlorite (compound) is a compound that contains this group, with chlorine in the oxidation state of +3. Chlorites are also known as salts of chlorous ac ...
and of kaolinite to illite at temperatures between . Because of these reactions, illite composes 80% of Precambrian
The Precambrian (or Pre-Cambrian, sometimes abbreviated pꞒ, or Cryptozoic) is the earliest part of Earth's history, set before the current Phanerozoic Eon. The Precambrian is so named because it preceded the Cambrian, the first period of the ...
shales, versus about 25% of young shales.
Unroofing of buried shale is accompanied by ''telogenesis'', the third and final stage of diagenesis. As erosion reduces the depth of burial, renewed exposure to meteoric water
Meteoric water is the water derived from precipitation (snow and rain). This includes water from lakes, rivers, and icemelts, which all originate from precipitation indirectly. While the bulk of rainwater or meltwater from snow and ice reaches the ...
produces additional changes to the shale, such as dissolution of some of the cement to produce secondary porosity
Porosity or void fraction is a measure of the void (i.e. "empty") spaces in a material, and is a fraction of the volume of voids over the total volume, between 0 and 1, or as a percentage between 0% and 100%. Strictly speaking, some tests measure ...
. Pyrite may be oxidized to produce gypsum
Gypsum is a soft sulfate mineral composed of calcium sulfate dihydrate, with the chemical formula . It is widely mined and is used as a fertilizer and as the main constituent in many forms of plaster, blackboard or sidewalk chalk, and drywall. ...
.
''Black shales'' are dark, as a result of being especially rich in unoxidized carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
. Common in some Paleozoic and Mesozoic
The Mesozoic Era ( ), also called the Age of Reptiles, the Age of Conifers, and colloquially as the Age of the Dinosaurs is the second-to-last era of Earth's geological history, lasting from about , comprising the Triassic, Jurassic and Cretaceo ...
strata
In geology and related fields, a stratum ( : strata) is a layer of rock or sediment characterized by certain lithologic properties or attributes that distinguish it from adjacent layers from which it is separated by visible surfaces known as ei ...
, black shales were deposited in anoxic
The term anoxia means a total depletion in the level of oxygen, an extreme form of hypoxia or "low oxygen". The terms anoxia and hypoxia are used in various contexts:
* Anoxic waters, sea water, fresh water or groundwater that are depleted of diss ...
, reducing environments, such as in stagnant water columns. Some black shales contain abundant heavy metals such as molybdenum
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin ''molybdaenum'', which is based on Ancient Greek ', meaning lead, since its ores were confused with lea ...
, uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
, vanadium
Vanadium is a chemical element with the symbol V and atomic number 23. It is a hard, silvery-grey, malleable transition metal. The elemental metal is rarely found in nature, but once isolated artificially, the formation of an oxide layer ( pas ...
, and zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
. The enriched values are of controversial origin, having been alternatively attributed to input from hydrothermal
Hydrothermal circulation in its most general sense is the circulation of hot water (Ancient Greek ὕδωρ, ''water'',Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon. revised and augmented throughout by Sir Henry Stuart Jones. with th ...
fluids during or after sedimentation or to slow accumulation from sea water
Seawater, or salt water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has approx ...
over long periods of sedimentation.
Karawanks
The Karawanks or Karavankas or Karavanks ( sl, Karavanke; german: Karawanken, ) are a mountain range of the Southern Limestone Alps on the border between Slovenia
Slovenia ( ; sl, Slovenija ), officially the Republic of Slovenia (Slovene: ...
, Austria
Austria, , bar, Östareich officially the Republic of Austria, is a country in the southern part of Central Europe, lying in the Eastern Alps. It is a federation of nine states, one of which is the capital, Vienna, the most populous ...
File:MesselShaleSplitting.JPG, Splitting shale ( Messel oil shale) with a large knife to reveal fossils
File:Shale 8040.jpg, Weathering
Weathering is the deterioration of rocks, soils and minerals as well as wood and artificial materials through contact with water, atmospheric gases, and biological organisms. Weathering occurs ''in situ'' (on site, with little or no movement), ...
shale at a road cut in southeastern Kentucky
Kentucky ( , ), officially the Commonwealth of Kentucky, is a state in the Southeastern region of the United States and one of the states of the Upper South. It borders Illinois, Indiana, and Ohio to the north; West Virginia and Virginia to ...
Fossil
A fossil (from Classical Latin , ) is any preserved remains, impression, or trace of any once-living thing from a past geological age. Examples include bones, shells, exoskeletons, stone imprints of animals or microbes, objects preserved ...
s, animal tracks or burrows and even raindrop impressions
Raindrop impressions are a geological feature characterized by small crater-like pits with slightly raised edges that are the result of the impact of Rain#Raindrop impacts, raindrop impacts on soft sediment surfaces.
Sedimentary structures with si ...
are sometimes preserved on shale bedding surfaces. Shales may also contain concretion
A concretion is a hard, compact mass of matter formed by the precipitation of mineral cement within the spaces between particles, and is found in sedimentary rock or soil. Concretions are often ovoid or spherical in shape, although irregular ...
s consisting of pyrite, apatite
Apatite is a group of phosphate minerals, usually hydroxyapatite, fluorapatite and chlorapatite, with high concentrations of OH−, F− and Cl− ions, respectively, in the crystal. The formula of the admixture of the three most common e ...
, or various carbonate minerals.
Shales that are subject to heat and pressure of metamorphism
Metamorphism is the transformation of existing rock (the protolith) to rock with a different mineral composition or texture. Metamorphism takes place at temperatures in excess of , and often also at elevated pressure or in the presence of chem ...
alter into a hard, fissile, metamorphic rock
Metamorphic rocks arise from the transformation of existing rock to new types of rock in a process called metamorphism. The original rock (protolith) is subjected to temperatures greater than and, often, elevated pressure of or more, causin ...
known as slate
Slate is a fine-grained, foliated, homogeneous metamorphic rock derived from an original shale-type sedimentary rock composed of clay or volcanic ash through low-grade regional metamorphism. It is the finest grained foliated metamorphic rock. ...
. With continued increase in metamorphic grade
Metamorphism is the transformation of existing rock (the protolith) to rock with a different mineral composition or texture. Metamorphism takes place at temperatures in excess of , and often also at elevated pressure or in the presence of che ...
the sequence is phyllite
Phyllite ( ) is a type of foliated metamorphic rock created from slate that is further metamorphosed so that very fine grained white mica achieves a preferred orientation.Stephen Marshak ''Essentials of Geology'', 3rd ed. It is primarily compo ...
, then schist
Schist ( ) is a medium-grained metamorphic rock showing pronounced schistosity. This means that the rock is composed of mineral grains easily seen with a low-power hand lens, oriented in such a way that the rock is easily split into thin flakes o ...
and finally gneiss
Gneiss ( ) is a common and widely distributed type of metamorphic rock. It is formed by high-temperature and high-pressure metamorphic processes acting on formations composed of igneous or sedimentary rocks. Gneiss forms at higher temperatures an ...
.
As hydrocarbon source rock
Shale is the most common source rock for hydrocarbons (natural gas
Natural gas (also called fossil gas or simply gas) is a naturally occurring mixture of gaseous hydrocarbons consisting primarily of methane in addition to various smaller amounts of other higher alkanes. Low levels of trace gases like carbo ...
and petroleum
Petroleum, also known as crude oil, or simply oil, is a naturally occurring yellowish-black liquid mixture of mainly hydrocarbons, and is found in geological formations. The name ''petroleum'' covers both naturally occurring unprocessed crud ...
). The lack of coarse sediments in most shale beds reflects the absence of strong currents in the waters of the depositional basin. These might have oxygenated the waters and destroyed organic matter before it could accumulate. The absence of carbonate rock in shale beds reflects the absence of organisms that might have secreted carbonate skeletons, also likely due to an anoxic environment. As a result, about 95% of organic matter in sedimentary rocks is found in shales and other mudrocks. Individual shale beds typically have an organic matter content of about 1%, but the richest source rocks may contain as much as 40% organic matter.
The organic matter in shale is converted over time from the original proteins, polysaccharides
Polysaccharides (), or polycarbohydrates, are the most abundant carbohydrates found in food. They are long chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with w ...
, lipids
Lipids are a broad group of naturally-occurring molecules which includes fats, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E and K), monoglycerides, diglycerides, phospholipids, and others. The functions of lipids include ...
, and other organic molecules to kerogen
Kerogen is solid, insoluble organic matter in sedimentary rocks. Comprising an estimated 1016 tons of carbon, it is the most abundant source of organic compounds on earth, exceeding the total organic content of living matter 10,000-fold. It ...
, which at the higher temperatures found at greater depths of burial is further converted to graphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on large ...
and petroleum.
Historical mining terminology
Before the mid-19th century, the termsslate
Slate is a fine-grained, foliated, homogeneous metamorphic rock derived from an original shale-type sedimentary rock composed of clay or volcanic ash through low-grade regional metamorphism. It is the finest grained foliated metamorphic rock. ...
, shale and schist
Schist ( ) is a medium-grained metamorphic rock showing pronounced schistosity. This means that the rock is composed of mineral grains easily seen with a low-power hand lens, oriented in such a way that the rock is easily split into thin flakes o ...
were not sharply distinguished. In the context of underground coal mining
Coal mining is the process of extracting coal from the ground. Coal is valued for its energy content and since the 1880s has been widely used to generate electricity. Steel and cement industries use coal as a fuel for extraction of iron from ...
, shale was frequently referred to as slate well into the 20th century. Black shale associated with coal seams is called black metal.
See also
* * * * * * * * * *References
External links
{{commonscatinline Industrial minerals