Scheele's Green on:
[Wikipedia]
[Google]
[Amazon]
 Scheele's Green, also called Schloss Green, is chemically a cupric hydrogen arsenite (also called copper arsenite or acidic copper arsenite), . It is chemically related to Paris Green. Scheele's Green was invented in 1775 by
Scheele's Green, also called Schloss Green, is chemically a cupric hydrogen arsenite (also called copper arsenite or acidic copper arsenite), . It is chemically related to Paris Green. Scheele's Green was invented in 1775 by
 Scheele's Green was used as a color for paper, e.g. for wallpapers and paper hangings, and in paints, wax candles, and even on some children's toys. It was also used to dye cotton and linen. Scheele's Green is more brilliant and durable than the then-used copper carbonate pigments. However, because of its copper content it tends to fade and blacken when exposed to
Scheele's Green was used as a color for paper, e.g. for wallpapers and paper hangings, and in paints, wax candles, and even on some children's toys. It was also used to dye cotton and linen. Scheele's Green is more brilliant and durable than the then-used copper carbonate pigments. However, because of its copper content it tends to fade and blacken when exposed to
Case Studies in Environmental Medicine – Arsenic Toxicity
Arsenites Copper(II) compounds Inorganic pigments Inorganic insecticides
 Scheele's Green, also called Schloss Green, is chemically a cupric hydrogen arsenite (also called copper arsenite or acidic copper arsenite), . It is chemically related to Paris Green. Scheele's Green was invented in 1775 by
Scheele's Green, also called Schloss Green, is chemically a cupric hydrogen arsenite (also called copper arsenite or acidic copper arsenite), . It is chemically related to Paris Green. Scheele's Green was invented in 1775 by Carl Wilhelm Scheele
Carl Wilhelm Scheele (, ; 9 December 1742 – 21 May 1786) was a Swedish German pharmaceutical chemist.
Scheele discovered oxygen (although Joseph Priestley published his findings first), and identified molybdenum, tungsten, barium, hydr ...
. By the end of the 19th century, it had virtually replaced the older green pigments based on copper carbonate Copper carbonate may refer to :
;Copper (II) compounds and minerals
* Copper(II) carbonate proper, (neutral copper carbonate): a rarely seen moisture-sensitive compound.
* Basic copper carbonate (the "copper carbonate" of commerce), actually a cop ...
. It is a yellowish-green pigment
A pigment is a colored material that is completely or nearly insoluble in water. In contrast, dyes are typically soluble, at least at some stage in their use. Generally dyes are often organic compounds whereas pigments are often inorganic compou ...
commonly used during the early to mid-19th century in paint
Paint is any pigmented liquid, liquefiable, or solid mastic composition that, after application to a substrate in a thin layer, converts to a solid film. It is most commonly used to protect, color, or provide texture. Paint can be made in many ...
s as well as being directly incorporated into a variety of products as a colorant. It began to fall out of favor after the 1860s because of its toxicity
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a subs ...
and the instability of its color in the presence of sulfide
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to chemical compounds la ...
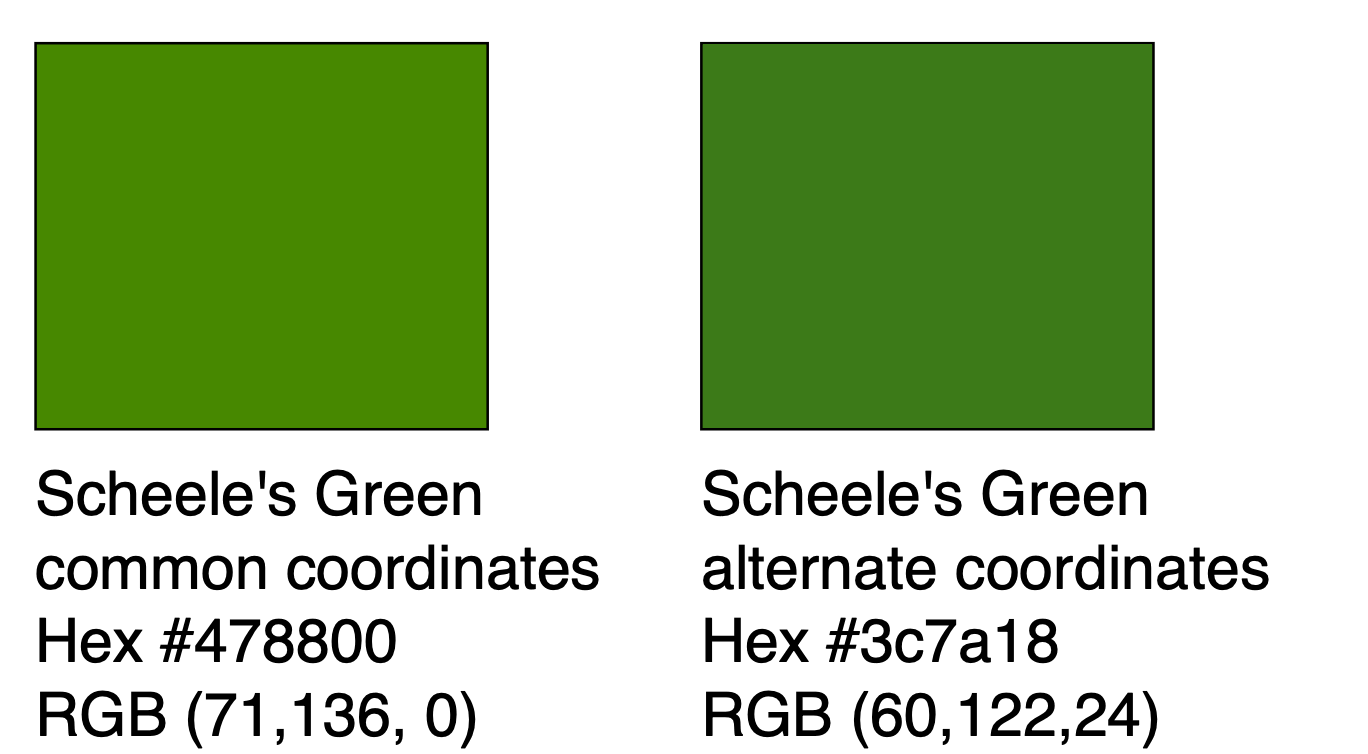
s and various chemical pollutants. The acutely toxic nature of Scheele's green as well as other arsenic-containing green pigments such as Paris Green may have contributed to the sharp decline in the popularity of the color green in late Victorian society. By the dawn of the 20th century, Scheele's green had completely fallen out of use as a pigment but was still in use as an insecticide into the 1930s. At least two modern reproductions of Scheele's green hue with modern non-toxic pigments have been made, with similar but non-identical color coordinates: one with hex#3c7a18 (RGB 60, 122, 24) and another with hex#478800 (RGB 71, 136, 0). The latter is the more typically reported color coordinate for Scheele's green.
Preparation
The pigment was originally prepared by making a solution ofsodium carbonate
Sodium carbonate, , (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield moderately alkaline solutions ...
at a temperature of around , then slowly adding arsenious oxide, while constantly stirring until everything had dissolved. This produced a sodium arsenite solution. Added to a copper sulfate Copper sulfate may refer to:
* Copper(II) sulfate, CuSO4, a common compound used as a fungicide and herbicide
* Copper(I) sulfate
Copper(I) sulfate, also known as cuprous sulfate, is an inorganic compound with the chemical formula Cu2 SO4. It ...
solution, it produced a green precipitate of effectively insoluble copper arsenite. After filtration the product was dried at about . To enhance the color, the salt was subsequently heated to . The intensity of the color depends on the copper : arsenic ratio, which in turn was affected by the ratio of the starting materials, as well as the temperature.
It has been found that Scheele's green was composed of a variety of different compounds, including copper metaarsenite (), copper arsenite salt ( and ), neutral copper orthoarsenite (), copper arsenate ( and ), and copper diarsenite ().
Uses
 Scheele's Green was used as a color for paper, e.g. for wallpapers and paper hangings, and in paints, wax candles, and even on some children's toys. It was also used to dye cotton and linen. Scheele's Green is more brilliant and durable than the then-used copper carbonate pigments. However, because of its copper content it tends to fade and blacken when exposed to
Scheele's Green was used as a color for paper, e.g. for wallpapers and paper hangings, and in paints, wax candles, and even on some children's toys. It was also used to dye cotton and linen. Scheele's Green is more brilliant and durable than the then-used copper carbonate pigments. However, because of its copper content it tends to fade and blacken when exposed to sulfide
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to chemical compounds la ...
s, whether in the form of atmospheric hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The under ...
or in pigment mixtures based on or containing sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formul ...
. Emerald green
Varieties of the color green may differ in hue, chroma (also called saturation or intensity) or lightness (or value, tone, or brightness), or in two or three of these qualities. Variations in value are also called tints and shades, a tint ...
, also known as Paris Green, was developed later in an attempt to improve Scheele's Green. It had the same tendency to blacken, but was more durable.
By the end of the 19th century, both greens were made obsolete by cobalt green, also known as zinc green, which is far less toxic.
Scheele's Green was used as an insecticide
Insecticides are substances used to kill insects. They include ovicides and larvicides used against insect eggs and larvae, respectively. Insecticides are used in agriculture, medicine, industry and by consumers. Insecticides are claimed t ...
in the 1930s, together with Paris Green.
Despite evidence of its high toxicity, Scheele's Green was also used as a food dye for sweets
Candy, also called sweets (British English) or lollies (Australian English, New Zealand English), is a confection that features sugar as a principal ingredient. The category, called ''sugar confectionery'', encompasses any sweet confection, i ...
such as green blancmange
Blancmange (, from french: blanc-manger ) is a sweet dessert popular throughout Europe commonly made with milk or cream and sugar thickened with rice flour, gelatin, corn starch, or Irish moss (a source of carrageenan), and often flavoured w ...
, a favorite of traders in 19th-century Greenock
Greenock (; sco, Greenock; gd, Grianaig, ) is a town and administrative centre in the Inverclyde council area in Scotland, United Kingdom and a former burgh within the historic county of Renfrewshire, located in the west central Lowland ...
; this led to a long-standing Scottish prejudice against green sweets.
Toxicity
In the 19th century, the toxicity of arsenic compounds was not readily known. Nineteenth-century journals contained reports of children wasting away in bright green rooms, of ladies in green dresses swooning, and of newspaper printers being overcome by arsenic vapors. There is one example of acute poisoning of children attending a Christmas party where dyed candles were burned. Although some European nations started banning arsenic-containing pigments in the 1830s and 1840s, Scheele's green did not completely fall out of favor until the 1860s. Publicity associated the 1861 death of 19-year-old Matilda Scheueur as a result of her job dusting artificial foliage with the pigment increased public awareness of the toxicity of Scheele's green. An article “Pretty Poison-Wreaths” described her repeated illness from arsenic poisoning leading to her death, and detailed autopsy findings of eyes and fingernails turned green from the pigment. By 1890's the last brand of wallpaper using it ceased production.Illness associated with arsenic containing wallpaper
Two main theories on the cause of wallpaper poisoning events have been proposed: dust particles caused by pigment and paper flaking, and toxic gas production. Tiny particles of the pigment can flake off and become airborne, and then are absorbed by the lungs. Alternatively, toxic gas can be released from compounds containing arsenic following certain chemical processes, such as heating, ormetabolism
Metabolism (, from el, μεταβολή ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run ...
by an organism. When the wallpaper becomes damp and moldy, the pigment may be metabolised, causing the release of poisonous arsine
Arsine (IUPAC name: arsane) is an inorganic compound with the formula As H3. This flammable, pyrophoric, and highly toxic pnictogen hydride gas is one of the simplest compounds of arsenic. Despite its lethality, it finds some applications ...
gas (). Fungi genera such as '' Scopulariopsis'' or '' Paecilomyces'' release arsine gas, when they are growing on a substance containing arsenic.
The Italian physician Bartolomeo Gosio published in 1893 his results on "Gosio gas", that was subsequently shown to contain trimethylarsine. Under wet conditions, the mold '' Scopulariopsis brevicaulis'' produced significant amounts of methyl arsines via methylation of arsenic-containing inorganic pigment
A pigment is a colored material that is completely or nearly insoluble in water. In contrast, dyes are typically soluble, at least at some stage in their use. Generally dyes are often organic compounds whereas pigments are often inorganic compou ...
s, especially Paris green and Scheele's Green.
In these compounds, the arsenic is either pentavalent or trivalent (arsenic is in group 15), depending on the compound. In humans, arsenic of these valences is readily absorbed by the gastrointestinal tract, which accounts for its high toxicity. Pentavalent arsenic tends to be reduced to trivalent arsenic and trivalent arsenic tends to proceed via oxidative methylation
In the chemical sciences, methylation denotes the addition of a methyl group on a substrate, or the substitution of an atom (or group) by a methyl group. Methylation is a form of alkylation, with a methyl group replacing a hydrogen atom. These ...
in which the trivalent arsenic is made into mono, di and trimethylated products by methyltransferases and an S-adenosyl-methionine
''S''-Adenosyl methionine (SAM), also known under the commercial names of SAMe, SAM-e, or AdoMet, is a common cosubstrate involved in methyl group transfers, transsulfuration, and aminopropylation. Although these anabolic reactions occur throug ...
methyl donating cofactor.
However, newer studies indicate that trimethylarsine has a low toxicity, and could therefore not account for the death and the severe health problems observed in the 19th century.
Arsenic is not only toxic, but it also has carcinogenic effects.
Role in Napoleon's death
During his exile on St. Helena,Napoleon
Napoleon Bonaparte ; it, Napoleone Bonaparte, ; co, Napulione Buonaparte. (born Napoleone Buonaparte; 15 August 1769 – 5 May 1821), later known by his regnal name Napoleon I, was a French military commander and political leader wh ...
resided in a house in which the rooms were painted bright green, his favorite color. The cause of his death is generally believed to have been stomach cancer, and arsenic exposure has been linked to an increased risk of gastric carcinoma. Analysis of samples of his hair revealed significant amounts of arsenic. As St. Helena has a rather damp climate, it is likely that fungus grew on the walls. It has also been suggested that the presence of such abnormally high levels of arsenic might be due to attempts at preserving his body.
See also
*Not to be confused withcopper arsenate
Copper arsenate (Cu3(AsO4)2·4H2O, or Cu5H2(AsO4)4·2H2O), also called copper orthoarsenate, tricopper arsenate, cupric arsenate, or tricopper orthoarsenate, is a blue or bluish-green powder insoluble in water and alcohol and soluble in aqueous ...
*List of inorganic pigments
The following list includes commercially or artistically important inorganic pigments of natural and synthetic origin..
Purple pigments
Aluminum pigments
* Ultramarine violet: (PV15) - a synthetic or naturally occurring sulfur containing silic ...
*''Shadows from the Walls of Death
''Shadows from the Walls of Death: Facts and Inferences Prefacing a Book of Specimens of Arsenical Wall Papers'' is an 1874 book by Dr. Robert C. Kedzie (1823–1902) of Michigan.
The book warns of the dangers of then commonly used arsenic-pigmen ...
''
References
{{reflist, 30emExternal links
Case Studies in Environmental Medicine – Arsenic Toxicity
Arsenites Copper(II) compounds Inorganic pigments Inorganic insecticides