synthetic genome on:
[Wikipedia]
[Google]
[Amazon]
 Artificial gene synthesis, or simply gene synthesis, refers to a group of methods that are used in
Artificial gene synthesis, or simply gene synthesis, refers to a group of methods that are used in
 The BioBricks assembly standard was described and introduced by Tom Knight in 2003 and it has been constantly updated since then. Currently, the most commonly used BioBricks standard is the assembly standard 10, or BBF
The BioBricks assembly standard was described and introduced by Tom Knight in 2003 and it has been constantly updated since then. Currently, the most commonly used BioBricks standard is the assembly standard 10, or BBF RFC 10 . BioBricks defines the prefix and suffix sequences required for a DNA part to be compatible with the BioBricks assembly method, allowing the joining of all DNA parts which are in the BioBricks format.
The prefix contains the restriction sites for EcoRI, NotI and XBaI, while the suffix contains the SpeI, NotI and PstI restriction sites. Outside of the prefix and suffix regions, the DNA part must not contain these restriction sites. To join two BioBrick parts together, one of the plasmids is digested with EcoRI and SpeI while the second plasmid is digested with EcoRI and XbaI. The two EcoRI overhangs are complementary and will thus anneal together, while SpeI and XbaI also produce complementary overhangs which can also be ligated together. As the resulting plasmid contains the original prefix and suffix sequences, it can be used to join with more BioBricks parts. Because of this property, the BioBricks assembly standard is said to be
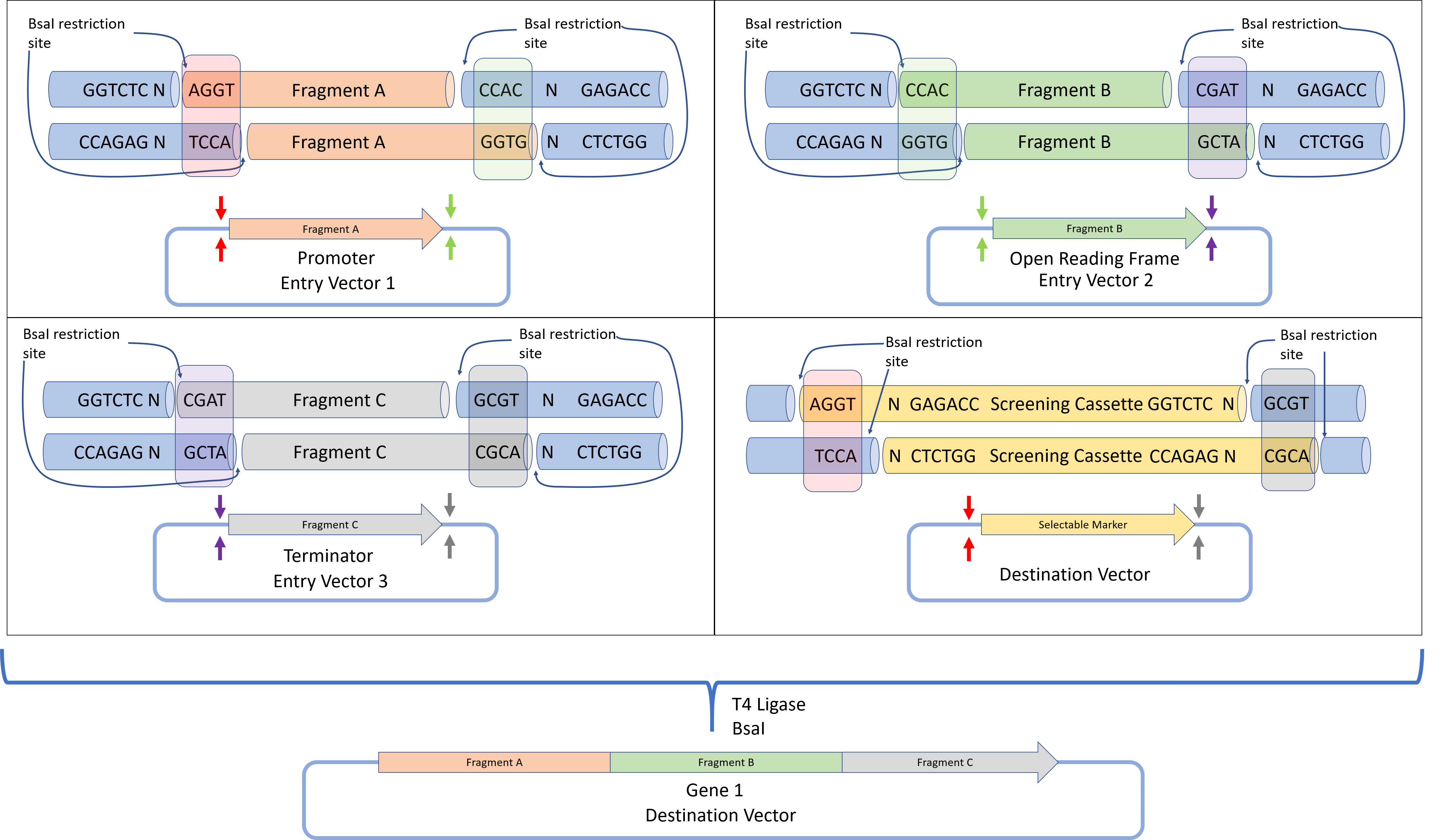 The Golden Gate assembly protocol was defined by Engler et al. 2008 to define a DNA assembly method that would give a final construct without a scar sequence, while also lacking the original restriction sites. This allows the protein to be expressed without containing unwanted protein sequences which could negatively affect protein folding or expression. By using the BsaI restriction enzyme that produces a 4 base pair overhang, up to 240 unique, non-palindromic sequences can be used for assembly.
Plasmid design and assembly
In Golden Gate cloning, each DNA fragment to be assembled is placed in a plasmid, flanked by inward facing BsaI restriction sites containing the programmed overhang sequences. For each DNA fragment, the 3' overhang sequence is complementary to the 5' overhang of the next downstream DNA fragment. For the first fragment, the 5' overhang is complementary to the 5' overhang of the destination plasmid, while the 3' overhang of the final fragment is complementary to the 3' overhang of the destination plasmid. Such a design allows for all DNA fragments to be assembled in a one-pot reaction (where all reactants are mixed together), with all fragments arranged in the correct sequence. Successfully assembled constructs are selected by detecting the loss of function of a screening cassette that was originally in the destination plasmid.
MoClo and Golden Braid
The original Golden Gate Assembly only allows for a single construct to be made in the destination vector . To enable this construct to be used in a subsequent reaction as an entry vector, the MoClo and Golden Braid standards were designed.
The MoClo standard involves defining multiple tiers of DNA assembly:
* Tier 1: Tier 1 assembly is the standard Golden Gate assembly, and genes are assembled from their components parts (DNA parts coding for genetic elements like UTRs, promoters, ribosome binding sites or
The Golden Gate assembly protocol was defined by Engler et al. 2008 to define a DNA assembly method that would give a final construct without a scar sequence, while also lacking the original restriction sites. This allows the protein to be expressed without containing unwanted protein sequences which could negatively affect protein folding or expression. By using the BsaI restriction enzyme that produces a 4 base pair overhang, up to 240 unique, non-palindromic sequences can be used for assembly.
Plasmid design and assembly
In Golden Gate cloning, each DNA fragment to be assembled is placed in a plasmid, flanked by inward facing BsaI restriction sites containing the programmed overhang sequences. For each DNA fragment, the 3' overhang sequence is complementary to the 5' overhang of the next downstream DNA fragment. For the first fragment, the 5' overhang is complementary to the 5' overhang of the destination plasmid, while the 3' overhang of the final fragment is complementary to the 3' overhang of the destination plasmid. Such a design allows for all DNA fragments to be assembled in a one-pot reaction (where all reactants are mixed together), with all fragments arranged in the correct sequence. Successfully assembled constructs are selected by detecting the loss of function of a screening cassette that was originally in the destination plasmid.
MoClo and Golden Braid
The original Golden Gate Assembly only allows for a single construct to be made in the destination vector . To enable this construct to be used in a subsequent reaction as an entry vector, the MoClo and Golden Braid standards were designed.
The MoClo standard involves defining multiple tiers of DNA assembly:
* Tier 1: Tier 1 assembly is the standard Golden Gate assembly, and genes are assembled from their components parts (DNA parts coding for genetic elements like UTRs, promoters, ribosome binding sites or


R2oDNA Designer
software and the overlap regions were designed to be 45 bp long to be compatible with Gibson assembly and other overlap assembly methods. To attach these linkers to the parts to be assembled, PCR is carried using part-specific primers containing 15 bp prefix and suffix adaptor sequences. The linkers are then attached to the adaptor sequences via a second PCR reaction. To position the DNA fragments, the same linker will be attached to the suffix of the desired upstream fragment and the prefix of the desired downstream fragments. Once the linkers are attached, Gibson assembly, CPEC, or the other overlap assembly methods can all be used to assemble the DNA fragments in the desired order.
Special Issue SYNTHETIC YEAST GENOME
''Science'' 10 March 2017 Vol 355, Issue 6329
 Artificial gene synthesis, or simply gene synthesis, refers to a group of methods that are used in
Artificial gene synthesis, or simply gene synthesis, refers to a group of methods that are used in synthetic biology
Synthetic biology (SynBio) is a multidisciplinary area of research that seeks to create new biological parts, devices, and systems, or to redesign systems that are already found in nature.
It is a branch of science that encompasses a broad ran ...
to construct and assemble gene
In biology, the word gene (from , ; "...Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth'' or ''gender'') can have several different meanings. The Mendelian gene is a ba ...
s from nucleotides '' de novo''. Unlike DNA synthesis in living cells, artificial gene synthesis does not require template DNA, allowing virtually any DNA sequence to be synthesized in the laboratory. It comprises two main steps, the first of which is solid-phase DNA synthesis, sometimes known as DNA printing. This produces oligonucleotide fragments that are generally under 200 base pairs. The second step then involves connecting these oligonucleotide fragments using various DNA assembly methods. Because artificial gene synthesis does not require template DNA, it is theoretically possible to make a completely synthetic DNA molecule with no limits on the nucleotide sequence or size.
Synthesis of the first complete gene, a yeast tRNA
Transfer RNA (abbreviated tRNA and formerly referred to as sRNA, for soluble RNA) is an adaptor molecule composed of RNA, typically 76 to 90 nucleotides in length (in eukaryotes), that serves as the physical link between the mRNA and the amino ac ...
, was demonstrated by Har Gobind Khorana
Har Gobind Khorana (9 January 1922 – 9 November 2011) was an Indian American biochemist. While on the faculty of the University of Wisconsin–Madison, he shared the 1968 Nobel Prize for Physiology or Medicine with Marshall W. Nirenberg and ...
and coworkers in 1972. Synthesis of the first peptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.
A ...
- and protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respo ...
-coding genes was performed in the laboratories of Herbert Boyer
Herbert Wayne "Herb" Boyer (born July 10, 1936) is an American biotechnologist, researcher and entrepreneur in biotechnology. Along with Stanley N. Cohen and Paul Berg he discovered a method to coax bacteria into producing foreign proteins, ther ...
and Alexander Markham, respectively. More recently, artificial gene synthesis methods have been developed that will allow the assembly of entire chromosomes and genomes. The first synthetic yeast
Yeasts are eukaryotic, single-celled microorganisms classified as members of the fungus kingdom. The first yeast originated hundreds of millions of years ago, and at least 1,500 species are currently recognized. They are estimated to constitut ...
chromosome was synthesised in 2014, and entire functional bacteria
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one biological cell. They constitute a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria were among ...
l chromosomes have also been synthesised. In addition, artificial gene synthesis could in the future make use of novel nucleobase
Nucleobases, also known as ''nitrogenous bases'' or often simply ''bases'', are nitrogen-containing biological compounds that form nucleosides, which, in turn, are components of nucleotides, with all of these monomers constituting the basic b ...
pairs (unnatural base pairs).
Standard methods for DNA synthesis
Oligonucleotide synthesis
Oligonucleotide
Oligonucleotides are short DNA or RNA molecules, oligomers, that have a wide range of applications in genetic testing, research, and forensics. Commonly made in the laboratory by solid-phase chemical synthesis, these small bits of nucleic acids c ...
s are chemically synthesized using building blocks called nucleoside phosphoramidite
A phosphoramidite (RO)2PNR2 is a monoamide of a phosphite diester. The key feature of phosphoramidites is their markedly high reactivity towards nucleophiles catalyzed by weak acids ''e.c''., triethylammonium chloride or 1''H''-tetrazole. In these ...
s. These can be normal or modified nucleosides which have protecting groups to prevent their amines, hydroxyl groups and phosphate groups from interacting incorrectly. One phosphoramidite is added at a time, the 5' hydroxyl group is deprotected and a new base is added and so on. The chain grows in the 3' to 5' direction, which is backwards relative to biosynthesis. At the end, all the protecting groups are removed. Nevertheless, being a chemical process, several incorrect interactions occur leading to some defective products. The longer the oligonucleotide sequence that is being synthesized, the more defects there are, thus this process is only practical for producing short sequences of nucleotides
Nucleotides are organic molecules consisting of a nucleoside and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecules w ...
. The current practical limit is about 200 bp (base pair
A base pair (bp) is a fundamental unit of double-stranded nucleic acids consisting of two nucleobases bound to each other by hydrogen bonds. They form the building blocks of the DNA double helix and contribute to the folded structure of both DNA ...
s) for an oligonucleotide with sufficient quality to be used directly for a biological application. HPLC
High-performance liquid chromatography (HPLC), formerly referred to as high-pressure liquid chromatography, is a technique in analytical chemistry used to separate, identify, and quantify each component in a mixture. It relies on pumps to pa ...
can be used to isolate products with the proper sequence. Meanwhile, a large number of oligos can be synthesized in parallel on gene chips. For optimal performance in subsequent gene synthesis procedures they should be prepared individually and in larger scales.
Annealing based connection of oligonucleotides
Usually, a set of individually designed oligonucleotides is made on automated solid-phase synthesizers, purified and then connected by specific annealing and standardligation
Ligation may refer to:
* Ligation (molecular biology), the covalent linking of two ends of DNA or RNA molecules
* In medicine, the making of a ligature (tie)
* Chemical ligation, the production of peptides from amino acids
* Tubal ligation, a meth ...
or polymerase
A polymerase is an enzyme ( EC 2.7.7.6/7/19/48/49) that synthesizes long chains of polymers or nucleic acids. DNA polymerase and RNA polymerase are used to assemble DNA and RNA molecules, respectively, by copying a DNA template strand using base- ...
reactions. To improve specificity of oligonucleotide annealing, the synthesis step relies on a set of thermostable DNA ligase
In biochemistry, a ligase is an enzyme that can catalyze the joining (ligation) of two large molecules by forming a new chemical bond. This is typically via hydrolysis of a small pendant chemical group on one of the larger molecules or the enzym ...
and polymerase
A polymerase is an enzyme ( EC 2.7.7.6/7/19/48/49) that synthesizes long chains of polymers or nucleic acids. DNA polymerase and RNA polymerase are used to assemble DNA and RNA molecules, respectively, by copying a DNA template strand using base- ...
enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
s. To date, several methods for gene synthesis have been described, such as the ligation of phosphorylated overlapping oligonucleotides, the Fok I method and a modified form of ligase chain reaction The ligase chain reaction (LCR) is a method of DNA amplification. The ligase chain reaction (LCR) is an amplification process that differs from PCR in that it involves a thermostable ligase to join two probes or other molecules together which can t ...
for gene synthesis. Additionally, several PCR assembly approaches have been described. They usually employ oligonucleotides of 40-50 nucleotides length that overlap each other. These oligonucleotides are designed to cover most of the sequence of both strands, and the full-length molecule is generated progressively by overlap extension (OE) PCR, thermodynamically balanced inside-out (TBIO) PCR or combined approaches. The most commonly synthesized genes range in size from 600 to 1,200 bp although much longer genes have been made by connecting previously assembled fragments of under 1,000 bp. In this size range it is necessary to test several candidate clones confirming the sequence of the cloned synthetic gene by automated sequencing methods.
Limitations
Moreover, because the assembly of the full-length gene product relies on the efficient and specific alignment of long single stranded oligonucleotides, critical parameters for synthesis success include extended sequence regions comprising secondary structures caused by inverted repeats, extraordinary high or low GC-content, or repetitive structures. Usually these segments of a particular gene can only be synthesized by splitting the procedure into several consecutive steps and a final assembly of shorter sub-sequences, which in turn leads to a significant increase in time and labor needed for its production. The result of a gene synthesis experiment depends strongly on the quality of the oligonucleotides used. For these annealing based gene synthesis protocols, the quality of the product is directly and exponentially dependent on the correctness of the employed oligonucleotides. Alternatively, after performing gene synthesis with oligos of lower quality, more effort must be made in downstream quality assurance during clone analysis, which is usually done by time-consuming standard cloning and sequencing procedures. Another problem associated with all current gene synthesis methods is the high frequency of sequence errors because of the usage of chemically synthesized oligonucleotides. The error frequency increases with longer oligonucleotides, and as a consequence the percentage of correct product decreases dramatically as more oligonucleotides are used. The mutation problem could be solved by shorter oligonucleotides used to assemble the gene. However, all annealing based assembly methods require the primers to be mixed together in one tube. In this case, shorter overlaps do not always allow precise and specific annealing of complementary primers, resulting in the inhibition of full length product formation. Manual design of oligonucleotides is a laborious procedure and does not guarantee the successful synthesis of the desired gene. For optimal performance of almost all annealing based methods, the melting temperatures of the overlapping regions are supposed to be similar for all oligonucleotides. The necessary primer optimisation should be performed using specialized oligonucleotide design programs. Several solutions for automated primer design for gene synthesis have been presented so far.Error correction procedures
To overcome problems associated with oligonucleotide quality several elaborate strategies have been developed, employing either separately prepared fishing oligonucleotides, mismatch binding enzymes of the mutS family or specific endonucleases from bacteria or phages. Nevertheless, all these strategies increase time and costs for gene synthesis based on the annealing of chemically synthesized oligonucleotides. Massively parallel sequencing has also been used as a tool to screen complex oligonucleotide libraries and enable the retrieval of accurate molecules. In one approach, oligonucleotides are sequenced on the 454 pyrosequencing platform and a robotic system images and picks individual beads corresponding to accurate sequence. In another approach, a complex oligonucleotide library is modified with unique flanking tags before massively parallel sequencing. Tag-directed primers then enable the retrieval of molecules with desired sequences by dial-out PCR. Increasingly, genes are ordered in sets including functionally related genes or multiple sequence variants on a single gene. Virtually all of the therapeutic proteins in development, such as monoclonal antibodies, are optimised by testing many gene variants for improved function or expression.Unnatural base pairs
While traditional nucleic acid synthesis only uses 4 base pairs - adenine, thymine, guanine and cytosine, oligonucleotide synthesis in the future could incorporate the use of unnatural base pairs, which are artificially designed and synthesized nucleobases that do not occur in nature. In 2012, a group of American scientists led by Floyd Romesberg, a chemical biologist at theScripps Research Institute
Scripps Research, previously known as The Scripps Research Institute (TSRI), is a nonprofit American medical research facility that focuses on research and education in the biomedical sciences. Headquartered in San Diego, California, the institu ...
in San Diego, California, published that his team designed an unnatural base pair (UBP). The two new artificial nucleotides or ''Unnatural Base Pair'' (UBP) were named d5SICS
d5SICS is an artificial nucleoside containing 6-methylisoquinoline-1-thione-2-yl group instead of a base.
It pairs up with dNaM in a hydrophobic interaction. It was not able to be removed by the error-correcting machinery of the '' E. coli'' into ...
and dNaM
dNaM is an artificial nucleobase containing a 3-methoxy-2-naphthyl group instead of a natural base.
When it was originally successfully introduced into DNA for replication in an E. coli semi-synthetic organism, it was paired up with d5SICS. Fo ...
. More technically, these artificial nucleotides
Nucleotides are organic molecules consisting of a nucleoside and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecules w ...
bearing hydrophobic nucleobase
Nucleobases, also known as ''nitrogenous bases'' or often simply ''bases'', are nitrogen-containing biological compounds that form nucleosides, which, in turn, are components of nucleotides, with all of these monomers constituting the basic b ...
s, feature two fused aromatic rings
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to saturat ...
that form a (d5SICS–dNaM) complex or base pair in DNA. In 2014 the same team from the Scripps Research Institute reported that they synthesized a stretch of circular DNA known as a plasmid
A plasmid is a small, extrachromosomal DNA molecule within a cell that is physically separated from chromosomal DNA and can replicate independently. They are most commonly found as small circular, double-stranded DNA molecules in bacteria; how ...
containing natural T-A and C-G base pairs along with the best-performing UBP Romesberg's laboratory had designed, and inserted it into cells of the common bacterium ''E. coli'' that successfully replicated the unnatural base pairs through multiple generations. This is the first known example of a living organism passing along an expanded genetic code to subsequent generations. This was in part achieved by the addition of a supportive algal gene that expresses a nucleotide triphosphate transporter which efficiently imports the triphosphates of both d5SICSTP and dNaMTP into ''E. coli'' bacteria. Then, the natural bacterial replication pathways use them to accurately replicate the plasmid
A plasmid is a small, extrachromosomal DNA molecule within a cell that is physically separated from chromosomal DNA and can replicate independently. They are most commonly found as small circular, double-stranded DNA molecules in bacteria; how ...
containing d5SICS–dNaM.
The successful incorporation of a third base pair is a significant breakthrough toward the goal of greatly expanding the number of amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha am ...
s which can be encoded by DNA, from the existing 20 amino acids to a theoretically possible 172, thereby expanding the potential for living organisms to produce novel protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respo ...
s. In the future, these unnatural base pairs could be synthesised and incorporated into oligonucleotides via DNA printing methods.
DNA assembly
DNA printing can thus be used to produce DNA parts, which are defined as sequences of DNA that encode a specific biological function (for example, promoters, transcription regulatory sequences oropen reading frame
In molecular biology, open reading frames (ORFs) are defined as spans of DNA sequence between the start and stop codons. Usually, this is considered within a studied region of a prokaryotic DNA sequence, where only one of the six possible readin ...
s). However, because oligonucleotide synthesis typically cannot accurately produce oligonucleotides sequences longer than a few hundred base pairs, DNA assembly methods have to be employed to assemble these parts together to create functional genes, multi-gene circuits or even entire synthetic chromosomes or genomes. Some DNA assembly techniques only define protocols for joining DNA parts, while other techniques also define the rules for the format of DNA parts that are compatible with them. These processes can be scaled up to enable the assembly of entire chromosomes or genomes. In recent years, there has been proliferation in the number of different DNA assembly standards with 14 different assembly standards developed as of 2015, each with their pros and cons. Overall, the development of DNA assembly standards has greatly facilitated the workflow of synthetic biology, aided the exchange of material between research groups and also allowed for the creation of modular and reusable DNA parts.
The various DNA assembly methods can be classified into three main categories – endonuclease-mediated assembly, site-specific recombination, and long-overlap-based assembly. Each group of methods has its distinct characteristics and their own advantages and limitations.
Endonuclease-mediated assembly
Endonuclease
Endonucleases are enzymes that cleave the phosphodiester bond within a polynucleotide chain. Some, such as deoxyribonuclease I, cut DNA relatively nonspecifically (without regard to sequence), while many, typically called restriction endonucleases ...
s are enzymes that recognise and cleave nucleic acid segments and they can be used to direct DNA assembly. Of the different types of restriction enzymes, the type II restriction enzymes are the most commonly available and used because their cleavage sites are located near or in their recognition sites. Hence, endonuclease-mediated assembly methods make use of this property to define DNA parts and assembly protocols.
BioBricks
 The BioBricks assembly standard was described and introduced by Tom Knight in 2003 and it has been constantly updated since then. Currently, the most commonly used BioBricks standard is the assembly standard 10, or BBF
The BioBricks assembly standard was described and introduced by Tom Knight in 2003 and it has been constantly updated since then. Currently, the most commonly used BioBricks standard is the assembly standard 10, or BBF idempotent
Idempotence (, ) is the property of certain operation (mathematics), operations in mathematics and computer science whereby they can be applied multiple times without changing the result beyond the initial application. The concept of idempotence ...
in nature. However, there will also be a "scar" sequence (either TACTAG or TACTAGAG) formed between the two fused BioBricks. This prevents BioBricks from being used to create fusion proteins, as the 6bp scar sequence codes for a tyrosine and a stop codon, causing translation to be terminated after the first domain is expressed, while the 8bp scar sequence causes a frameshift
Ribosomal frameshifting, also known as translational frameshifting or translational recoding, is a biological phenomenon that occurs during translation that results in the production of multiple, unique proteins from a single mRNA. The process can ...
, preventing continuous readthrough of the codons. To offer alternative scar sequences that for example give a 6bp scar, or scar sequences that do not contain stop codons, other assembly standards such as the BB-2 Assembly, BglBricks Assembly, Silver Assembly and the Freiburg Assembly were designed.
While the easiest method to assemble BioBrick parts is described above, there also exist several other commonly used assembly methods that offer several advantages over the standard assembly. The 3 antibiotic (3A) assembly allows for the correct assembly to be selected via antibiotic selection, while the amplified insert assembly seeks to overcome the low transformation efficiency seen in 3A assembly.
The BioBrick assembly standard has also served as inspiration for using other types of endonucleases for DNA assembly. For example, both the iBrick standard and the HomeRun vector assembly standards employ homing endonucleases instead of type II restriction enzymes.
Type IIs restriction endonuclease assembly
Some assembly methods also make use of type IIs restriction endonucleases. These differ from other type II endonucleases as they cut several base pairs away from the recognition site. As a result, the overhang sequence can be modified to contain the desired sequence. This provides Type IIs assembly methods with two advantages – it enables "scar-less" assembly, and allows for one-pot, multi-part assembly. Assembly methods that use type IIs endonucleases includeGolden Gate
The Golden Gate is a strait on the west coast of North America that connects San Francisco Bay to the Pacific Ocean. It is defined by the headlands of the San Francisco Peninsula and the Marin Peninsula, and, since 1937, has been spanned by th ...
and its associated variants.
= Golden Gate cloning
= The Golden Gate assembly protocol was defined by Engler et al. 2008 to define a DNA assembly method that would give a final construct without a scar sequence, while also lacking the original restriction sites. This allows the protein to be expressed without containing unwanted protein sequences which could negatively affect protein folding or expression. By using the BsaI restriction enzyme that produces a 4 base pair overhang, up to 240 unique, non-palindromic sequences can be used for assembly.
Plasmid design and assembly
In Golden Gate cloning, each DNA fragment to be assembled is placed in a plasmid, flanked by inward facing BsaI restriction sites containing the programmed overhang sequences. For each DNA fragment, the 3' overhang sequence is complementary to the 5' overhang of the next downstream DNA fragment. For the first fragment, the 5' overhang is complementary to the 5' overhang of the destination plasmid, while the 3' overhang of the final fragment is complementary to the 3' overhang of the destination plasmid. Such a design allows for all DNA fragments to be assembled in a one-pot reaction (where all reactants are mixed together), with all fragments arranged in the correct sequence. Successfully assembled constructs are selected by detecting the loss of function of a screening cassette that was originally in the destination plasmid.
MoClo and Golden Braid
The original Golden Gate Assembly only allows for a single construct to be made in the destination vector . To enable this construct to be used in a subsequent reaction as an entry vector, the MoClo and Golden Braid standards were designed.
The MoClo standard involves defining multiple tiers of DNA assembly:
* Tier 1: Tier 1 assembly is the standard Golden Gate assembly, and genes are assembled from their components parts (DNA parts coding for genetic elements like UTRs, promoters, ribosome binding sites or
The Golden Gate assembly protocol was defined by Engler et al. 2008 to define a DNA assembly method that would give a final construct without a scar sequence, while also lacking the original restriction sites. This allows the protein to be expressed without containing unwanted protein sequences which could negatively affect protein folding or expression. By using the BsaI restriction enzyme that produces a 4 base pair overhang, up to 240 unique, non-palindromic sequences can be used for assembly.
Plasmid design and assembly
In Golden Gate cloning, each DNA fragment to be assembled is placed in a plasmid, flanked by inward facing BsaI restriction sites containing the programmed overhang sequences. For each DNA fragment, the 3' overhang sequence is complementary to the 5' overhang of the next downstream DNA fragment. For the first fragment, the 5' overhang is complementary to the 5' overhang of the destination plasmid, while the 3' overhang of the final fragment is complementary to the 3' overhang of the destination plasmid. Such a design allows for all DNA fragments to be assembled in a one-pot reaction (where all reactants are mixed together), with all fragments arranged in the correct sequence. Successfully assembled constructs are selected by detecting the loss of function of a screening cassette that was originally in the destination plasmid.
MoClo and Golden Braid
The original Golden Gate Assembly only allows for a single construct to be made in the destination vector . To enable this construct to be used in a subsequent reaction as an entry vector, the MoClo and Golden Braid standards were designed.
The MoClo standard involves defining multiple tiers of DNA assembly:
* Tier 1: Tier 1 assembly is the standard Golden Gate assembly, and genes are assembled from their components parts (DNA parts coding for genetic elements like UTRs, promoters, ribosome binding sites or terminator
Terminator may refer to:
Science and technology
Genetics
* Terminator (genetics), the end of a gene for transcription
* Terminator technology, proposed methods for restricting the use of genetically modified plants by causing second generation s ...
sequences). Flanking the insertion site of the tier 1 destination vectors are a pair of inward cutting BpiI restriction sites. This allows these plasmids to be used as entry vectors for tier two destination vectors.
* Tier 2: Tier 2 assembly involves further assembling the genes assembled in tier 1 assembly into multi-gene constructs. If there is a need for further, higher tier assembly, inward cutting BsaI restriction sites can be added to flank the insertion sites. These vectors can then be used as entry vectors for higher tier constructs.
Each assembly tier alternates the use of BsaI and BpiI restriction sites to minimise the number of forbidden sites, and sequential assembly for each tier is achieved by following the Golden Gate plasmid design. Overall, the MoClo standard allows for the assembly of a construct that contains multiple transcription units, all assembled from different DNA parts, by a series of one-pot Golden Gate reactions. However, one drawback of the MoClo standard is that it requires the use of 'dummy parts' with no biological function, if the final construct requires less than four component parts. The Golden Braid standard on the other hand introduced a pairwise Golden Gate assembly standard.
The Golden Braid standard uses the same tiered assembly as MoClo, but each tier only involves the assembly of two DNA fragments, i.e. a pairwise approach. Hence in each tier, pairs of genes are cloned into a destination fragment in the desired sequence, and these are subsequently assembled two at a time in successive tiers. Like MoClo, the Golden Braid standard alternates the BsaI and BpiI restriction enzymes between each tier.
The development of the Golden Gate assembly methods and its variants has allowed researchers to design tool-kits to speed up the synthetic biology workflow. For example, EcoFlex was developed as a toolkit for ''E. Coli'' that uses the MoClo standard for its DNA parts, while a similar toolkit has also been developed for engineering the ''Chlamydomonas reinhardtii'' microalgae.
Site-specific recombination
Site-specific recombination makes use of phageintegrase
Retroviral integrase (IN) is an enzyme produced by a retrovirus (such as HIV) that integrates—forms covalent links between—its genetic information into that of the host cell it infects. Retroviral INs are not to be confused with phage int ...
s instead of restriction enzymes, eliminating the need for having restriction sites in the DNA fragments. Instead, integrases make use of unique attachment (att) sites, and catalyse DNA rearrangement between the target fragment and the destination vector. The Invitrogen Gateway cloning system was invented in the late 1990s and uses two proprietary enzyme mixtures, BP clonase and LR clonase. The BP clonase mix catalyses the recombination between attB and attP sites, generating hybrid attL and attR sites, while the LR clonase mix catalyse the recombination of attL and attR sites to give attB and attP sites. As each enzyme mix recognises only specific att sites, recombination is highly specific and the fragments can be assembled in the desired sequence.
Vector design and assembly
Because Gateway cloning is a proprietary technology, all Gateway reactions must be carried out with the Gateway kit that is provided by the manufacturer. The reaction can be summarised into two steps. The first step involves assembling the entry clones containing the DNA fragment of interest, while the second step involves inserting this fragment of interest into the destination clone.
# Entry clones must be made using the supplied "Donor" vectors containing a Gateway cassette flanked by attP sites. The Gateway cassette contains a bacterial suicide gene (e.g. ccdB
Christian Commission for Development in Bangladesh (CCDB) founded in 1972, immediately after the Bangladesh Liberation War, by the World Council of Churches (WCC) to succeed the Bangladesh Ecumenical Relief and Rehabilitation Services (BERRS). Th ...
) that will allow for survival and selection of successfully recombined entry clones. A pair of attB sites are added to flank the DNA fragment of interest, and this will allow recombination with the attP sites when the BP clonase mix is added. Entry clones are produced, and the fragment of interest is flanked by attL sites.
# The destination vector also comes with a Gateway cassette, but is instead flanked by a pair of attR sites. Mixing this destination plasmid with the entry clones and the LR clonase mix will allow for recombination to occur between the attR and attL sites. A destination clone is produced, with the fragment of interest successfully inserted. The lethal gene is inserted into the original vector, and bacteria transformed with this plasmid will die. The desired vector can thus be easily selected.
The earliest iterations of the Gateway cloning method only allowed for only one entry clone to be used for each destination clone produced. However, further research revealed that four more orthogonal att sequences could be generated, allowing for the assembly of up to four different DNA fragments, and this process is now known as the Multisite Gateway technology.
Besides Gateway cloning, non-commercial methods using other integrases have also been developed. For example, the Serine Integrase Recombinational Assembly (SIRA) method uses the ϕC31 integrase, while the Site-Specific Recombination-based Tandem Assembly (SSRTA) method uses the ''Streptomyces'' phage φBT1 integrase. Other methods, like the HomeRun Vector Assembly System (HVAS), build on the Gateway cloning system and further incorporate homing endonucleases to design a protocol that could potentially support the industrial synthesis of synthetic DNA constructs.

Long-overlap-based assembly
There have been a variety of long-overlap-based assembly methods developed in recent years. One of the most commonly used methods, the Gibson assembly method, was developed in 2009, and provides a one-pot DNA assembly method that does not require the use of restriction enzymes or integrases. Other similar overlap-based assembly methods include Circular Polymerase Extension Cloning (CPEC), Sequence and Ligase Independent Cloning (SLIC) and Seamless Ligation Cloning Extract (SLiCE). Despite the presence of many overlap assembly methods, the Gibson assembly method is still the most popular. Besides the methods listed above, other researchers have built on the concepts used in Gibson assembly and other assembly methods to develop new assembly strategies like the Modular Overlap-Directed Assembly with Linkers (MODAL) strategy, or the Biopart Assembly Standard for Idempotent Cloning (BASIC) method.Gibson assembly
The Gibson assembly method is a relatively straightforward DNA assembly method, requiring only a few additional reagents: the 5' T5exonuclease
Exonucleases are enzymes that work by cleaving nucleotides one at a time from the end (exo) of a polynucleotide chain. A hydrolyzing reaction that breaks phosphodiester bonds at either the 3′ or the 5′ end occurs. Its close relative is the ...
, Phusion DNA polymerase
A DNA polymerase is a member of a family of enzymes that catalyze the synthesis of DNA molecules from nucleoside triphosphates, the molecular precursors of DNA. These enzymes are essential for DNA replication and usually work in groups to create ...
, and Taq Taq may refer to:
* Taq, Iran, a village in Semnan Province, Iran
* ''Taq'' polymerase, a heat-stable enzyme used in polymerase chain reaction, or ''Thermus aquaticus'', the species of bacteria from which the polymerase is naturally derived
* The ...
DNA ligase
DNA ligase is a specific type of enzyme, a ligase, () that facilitates the joining of DNA strands together by catalyzing the formation of a phosphodiester bond. It plays a role in repairing single-strand breaks in duplex DNA in living organ ...
. The DNA fragments to be assembled are synthesised to have overlapping 5' and 3' ends in the order that they are to be assembled in. These reagents are mixed together with the DNA fragments to be assembled at 50 °C and the following reactions occur:
# The T5 exonuclease chews back DNA from the 5' end of each fragment, exposing 3' overhangs on each DNA fragment.
# The complementary overhangs on adjacent DNA fragments anneal via complementary base pairing.
# The Phusion DNA polymerase fills in any gaps where the fragments anneal.
# Taq DNA ligase repairs the nicks on both DNA strands.
Because the T5 exonuclease is heat labile, it is inactivated at 50 °C after the initial chew back step. The product is thus stable, and the fragments assembled in the desired order. This one-pot protocol can assemble up to 5 different fragments accurately, while several commercial providers have kits to accurately assemble up to 15 different fragments in a two-step reaction. However, while the Gibson assembly protocol is fast and uses relatively few reagents, it requires bespoke DNA synthesis as each fragment has to be designed to contain overlapping sequences with the adjacent fragments and amplified via PCR. This reliance on PCR may also affect the fidelity of the reaction when long fragments, fragments with high GC content or repeat sequences are used.

MODAL
The MODAL strategy defines overlap sequences known as "linkers" to reduce the amount of customisation that needs to be done with each DNA fragment. The linkers were designed using thR2oDNA Designer
software and the overlap regions were designed to be 45 bp long to be compatible with Gibson assembly and other overlap assembly methods. To attach these linkers to the parts to be assembled, PCR is carried using part-specific primers containing 15 bp prefix and suffix adaptor sequences. The linkers are then attached to the adaptor sequences via a second PCR reaction. To position the DNA fragments, the same linker will be attached to the suffix of the desired upstream fragment and the prefix of the desired downstream fragments. Once the linkers are attached, Gibson assembly, CPEC, or the other overlap assembly methods can all be used to assemble the DNA fragments in the desired order.
BASIC
The BASIC assembly strategy was developed in 2015 and sought to address the limitations of previous assembly techniques, incorporating six key concepts from them: standard reusable parts; single-tier format (all parts are in the same format and are assembled using the same process); idempotent cloning; parallel (multipart) DNA assembly; size independence; automatability. DNA parts and linker design The DNA parts are designed and cloned into storage plasmids, with the part flanked by an integrated prefix (''i''P) and an integrated suffix (''i''S) sequence. The ''i''P and ''i''S sequences contain inward facing BsaI restriction sites, which contain overhangs complementary to the BASIC linkers. Like in MODAL, the 7 standard linkers used in BASIC were designed with the R2oDNA Designer software, and screened to ensure that they do not contain sequences with homology to chassis genomes, and that they do not contain unwanted sequences like secondary structure sequences, restriction sites or ribosomal binding sites. Each linker sequence is split into two halves, each with a 4 bp overhang complementary to the BsaI restriction site, a 12 bp double stranded sequence and sharing a 21 bp overlap sequence with the other half. The half that is will bind to the upstream DNA part is known as the suffix linker part (e.g. L1S) and the half that binds to the downstream part is known as the prefix linker part (e.g. L1P). These linkers form the basis of assembling the DNA parts together. Besides directing the order of assembly, the standard BASIC linkers can also be modified to carry out other functions. To allow for idempotent assembly, linkers were also designed with additional methylated ''i''P and ''i''S sequences inserted to protect them from being recognised by BsaI. This methylation is lost following transformation and in vivo plasmid replication, and the plasmids can be extracted, purified, and used for further reactions. Because the linker sequence are relatively long (45bp for a standard linker), there is an opportunity to incorporate functional DNA sequences to reduce the number of DNA parts needed during assembly. The BASIC assembly standard provides several linkers embedded with RBS of different strengths. Similarly to facilitate the construction of fusion proteins containing multiple protein domains, several fusion linkers were also designed to allow for full read-through of the DNA construct. These fusion linkers code for a 15 amino acid glycine and serine polypeptide, which is an ideal linker peptide for fusion proteins with multiple domains. Assembly There are three main steps in the assembly of the final construct. # First, the DNA parts are excised from the storage plasmid, giving a DNA fragment with BsaI overhangs on the 3' and 5' end. # Next, each linker part is attached to its respective DNA part by incubating with T4 DNA ligase. Each DNA part will have a suffix and prefix linker part from two different linkers to direct the order of assembly. For example, the first part in the sequence will have L1P and L2S, while the second part will have L2P and L3S attached. The linker parts can be changed to change the sequence of assembly. # Finally, the parts with the attached linkers are assembled into a plasmid by incubating at 50 °C. The 21 bp overhangs of the P and S linkers anneal and the final construct can be transformed into bacteria cells for cloning. The single stranded nicks are repaired ''in vivo'' following transformation, producing a stable final construct cloned into plasmids.Applications
As DNA printing and DNA assembly methods have allowed commercial gene synthesis to become progressively and exponentially cheaper over the past years, artificial gene synthesis represents a powerful and flexible engineering tool for creating and designing new DNA sequences and protein functions. Besides synthetic biology, various research areas like those involving heterologous gene expression,vaccine
A vaccine is a biological Dosage form, preparation that provides active acquired immunity to a particular infectious disease, infectious or cancer, malignant disease. The safety and effectiveness of vaccines has been widely studied and verifie ...
development, gene therapy
Gene therapy is a medical field which focuses on the genetic modification of cells to produce a therapeutic effect or the treatment of disease by repairing or reconstructing defective genetic material. The first attempt at modifying human DN ...
and molecular engineering, would benefit greatly from having fast and cheap methods to synthesise DNA to code for proteins and peptides. The methods used for DNA printing and assembly have even enabled the use of DNA as an information storage medium.
Synthesising bacterial genomes
Synthia and ''Mycoplasma laboratorium''
On June 28, 2007, a team at the J. Craig Venter Institute published an article in ''Science Express'', saying that they had successfully transplanted the natural DNA from a ''Mycoplasma mycoides
''Mycoplasma mycoides'' is a bacterial species of the genus ''Mycoplasma'' in the class Mollicutes.
This microorganism is a parasite that lives in ruminants. ''Mycoplasma mycoides'' comprises two subspecies, '' mycoides'' and ''capri'', which in ...
'' bacterium into a ''Mycoplasma capricolum
''Mycoplasma capricolum'' is a species of Mycoplasma bacteria. It is primarily a pathogen of goat
The goat or domestic goat (''Capra hircus'') is a domesticated species of goat-antelope typically kept as livestock. It was domesticated fro ...
'' cell, creating a bacterium which behaved like a ''M. mycoides''.
On Oct 6, 2007, Craig Venter
John Craig Venter (born October 14, 1946) is an American biotechnologist and businessman. He is known for leading one of the first draft sequences of the human genome and assembled the first team to transfect a cell with a synthetic chromosome. ...
announced in an interview with UK's ''The Guardian
''The Guardian'' is a British daily newspaper. It was founded in 1821 as ''The Manchester Guardian'', and changed its name in 1959. Along with its sister papers ''The Observer'' and ''The Guardian Weekly'', ''The Guardian'' is part of the Gu ...
'' newspaper that the same team had synthesized a modified version of the single chromosome
A chromosome is a long DNA molecule with part or all of the genetic material of an organism. In most chromosomes the very long thin DNA fibers are coated with packaging proteins; in eukaryotic cells the most important of these proteins are ...
of ''Mycoplasma genitalium
''Mycoplasma genitalium'' (''MG'', commonly known as Mgen) is a sexually transmitted, small and pathogenic bacterium that lives on the mucous epithelial cells of the urinary and genital tracts in humans. Medical reports published in 2007 and 201 ...
'' artificially. The chromosome was modified to eliminate all genes which tests in live bacteria had shown to be unnecessary. The next planned step in this ''minimal genome project'' is to transplant the synthesized minimal genome into a bacterial cell with its old DNA removed; the resulting bacterium will be called ''Mycoplasma laboratorium
''Mycoplasma laboratorium'' or Synthia refers to a synthetic strain of bacterium. The project to build the new bacterium has evolved since its inception. Initially the goal was to identify a minimal set of genes that are required to sustain lif ...
''. The next day the Canadian bioethics
Bioethics is both a field of study and professional practice, interested in ethical issues related to health (primarily focused on the human, but also increasingly includes animal ethics), including those emerging from advances in biology, med ...
group, ETC Group issued a statement through their representative, Pat Mooney
Pat Roy Mooney, for more than thirty years, has worked with civil society organizations on international trade and development issues related to agriculture, biodiversity and emerging technologies.
He was born and lived on the Canadian prairies f ...
, saying Venter's "creation" was "a chassis on which you could build almost anything". The synthesized genome had not yet been transplanted into a working cell.
On May 21, 2010, ''Science'' reported that the Venter group had successfully synthesized the genome of the bacterium ''Mycoplasma mycoides'' from a computer record, and transplanted the synthesized genome into the existing cell of a ''Mycoplasma capricolum'' bacterium that had its DNA removed. The "synthetic" bacterium was viable, i.e. capable of replicating billions of times. The team had originally planned to use the ''M. genitalium'' bacterium they had previously been working with, but switched to ''M. mycoides'' because the latter bacterium grows much faster, which translated into quicker experiments. Venter describes it as "the first species.... to have its parents be a computer". The transformed bacterium is dubbed "Synthia
''Mycoplasma laboratorium'' or Synthia refers to a synthetic strain of bacterium. The project to build the new bacterium has evolved since its inception. Initially the goal was to identify a minimal set of genes that are required to sustain lif ...
" by ETC. A Venter spokesperson has declined to confirm any breakthrough at the time of this writing.
Synthetic Yeast 2.0
As part of the Synthetic Yeast 2.0 project, various research groups around the world have participated in a project to synthesise synthetic yeast genomes, and through this process, optimise the genome of the model organism ''Saccharomyces cerevisiae
''Saccharomyces cerevisiae'' () (brewer's yeast or baker's yeast) is a species of yeast (single-celled fungus microorganisms). The species has been instrumental in winemaking, baking, and brewing since ancient times. It is believed to have been o ...
''. The Yeast 2.0 project applied various DNA assembly methods that have been discussed above, and in March 2014, Jef Boeke
Jef D. Boeke is an American geneticist who is currently the founding director of The Institute for Systems Genetics at NYU Langone Medical Center. From 1986 to 2014 he was on the faculty of The Johns Hopkins University School of Medicine, where h ...
of the Langone Medical Centre at New York University, revealed that his team had synthesized chromosome III of ''S. cerevisiae''. The procedure involved replacing the genes in the original chromosome with synthetic versions and the finished synthetic chromosome was then integrated into a yeast cell. It required designing and creating 273,871 base pairs of DNA – fewer than the 316,667 pairs in the original chromosome. In March 2017, the synthesis of 6 of the 16 chromosomes had been completed, with synthesis of the others still ongoing.''Science'' 10 March 2017 Vol 355, Issue 6329
See also
*DNA sequencing
DNA sequencing is the process of determining the nucleic acid sequence – the order of nucleotides in DNA. It includes any method or technology that is used to determine the order of the four bases: adenine, guanine, cytosine, and thymine. Th ...
* Genetic modification
Genetic engineering, also called genetic modification or genetic manipulation, is the modification and manipulation of an organism's genes using technology. It is a set of technologies used to change the genetic makeup of cells, including t ...
* Protein engineering
Protein engineering is the process of developing useful or valuable proteins. It is a young discipline, with much research taking place into the understanding of protein folding and recognition for protein design principles. It has been used to imp ...
* Synthetic Gene Database
Notes
{{DEFAULTSORT:Gene Synthesis (artificial) Chemical synthesis Gene expression Genetically modified organisms Genetics techniques Molecular genetics Protein biosynthesis Synthetic biology