Synaptogenesis is the formation of
synapse
In the nervous system, a synapse is a structure that allows a neuron (or nerve cell) to pass an electrical or chemical signal to another neuron or a target effector cell. Synapses can be classified as either chemical or electrical, depending o ...
s between
neuron
A neuron (American English), neurone (British English), or nerve cell, is an membrane potential#Cell excitability, excitable cell (biology), cell that fires electric signals called action potentials across a neural network (biology), neural net ...
s in the
nervous system
In biology, the nervous system is the complex system, highly complex part of an animal that coordinates its behavior, actions and sense, sensory information by transmitting action potential, signals to and from different parts of its body. Th ...
. Although it occurs throughout a healthy person's
life
Life, also known as biota, refers to matter that has biological processes, such as Cell signaling, signaling and self-sustaining processes. It is defined descriptively by the capacity for homeostasis, Structure#Biological, organisation, met ...
span, an explosion of synapse formation occurs during early
brain development
The brain is an organ that serves as the center of the nervous system in all vertebrate and most invertebrate animals. It consists of nervous tissue and is typically located in the head ( cephalization), usually near organs for special sens ...
, known as exuberant synaptogenesis.
Synaptogenesis is particularly important during an individual's
critical period
In developmental psychology and developmental biology, a critical period is a maturational stage in the lifespan of an organism during which the nervous system is especially sensitive to certain environmental stimuli. If, for some reason, the org ...
, during which there is a certain degree of
synaptic pruning
Synaptic pruning is the process of synapse elimination or weakening. Though it occurs throughout the lifespan of a mammal, the most active period of synaptic pruning in the development of the nervous system occurs between early childhood and the o ...
due to competition for
neural growth factors by neurons and synapses. Processes that are not used, or inhibited during their critical period will fail to develop normally later on in life.
Exuberant synaptogenesis
Brain growth and development begins during gestation and into the postnatal period. Brain development can be divided into stages including:
neurogenesis
Neurogenesis is the process by which nervous system cells, the neurons, are produced by neural stem cells (NSCs). This occurs in all species of animals except the porifera (sponges) and placozoans. Types of NSCs include neuroepithelial cells ( ...
, differentiation, proliferation, migration, synaptogenesis,
gliogenesis and
myelination, and
apoptosis
Apoptosis (from ) is a form of programmed cell death that occurs in multicellular organisms and in some eukaryotic, single-celled microorganisms such as yeast. Biochemistry, Biochemical events lead to characteristic cell changes (Morphology (biol ...
and
synaptic pruning
Synaptic pruning is the process of synapse elimination or weakening. Though it occurs throughout the lifespan of a mammal, the most active period of synaptic pruning in the development of the nervous system occurs between early childhood and the o ...
. Synaptogenesis occurs in the third trimester during gestation as well as the first two years postnatal.
During neuron differentiation,
growth cones
Growth may refer to:
Biology
*Auxology, the study of all aspects of human physical growth
*Bacterial growth
*Cell growth
*Growth hormone, a peptide hormone that stimulates growth
*Human development (biology)
*Plant growth
*Secondary growth, growt ...
that extend off the tip of each axon act as the site for elongation of each axon. These growth cones find signal molecules which act as guidance cues and form synapses. Connections formed between
neurites may be random or selective.
Exuberant synaptogenesis is characterized by a few characteristics. First, it involves the formation of long axonal projections, and an overproduction of small axonal branches, synapses, and dendritic branches and/or
spines. Throughout this process, many of these structures may be maintained or eventually eliminated. Elimination may occur by neuronal death or selective deletion.
Developmental exuberance may occur macro- or microscopically. Macroscopic exuberance occurs when transient projections are formed between macroscopic regions in the brain. In comparison, microscopic exuberance occurs when transient structures involved in communication between neurons forms.
Signaling molecules
UNC-4 transcription factor
What specific molecules and chemical signals are involved in synaptogenesis has yet to be fully understood. Some evidence posits that
transcription factors
In molecular biology, a transcription factor (TF) (or sequence-specific DNA-binding factor) is a protein that controls the rate of transcription of genetic information from DNA to messenger RNA, by binding to a specific DNA sequence. The fun ...
are heavily involved in directing where axons and dendrites form synapses before and after synaptogenesis. The main study focusing on this involved motor neurons of ''
C.elegans''. In this study, researchers found that knockout animals without the gene, ''unc-4'' have motor defects specifically with moving backwards. This gene is necessary for the Prd-like homeodomain transcription factor. These animals also had abnormal synaptic specificity indicating that this transcription factor is likely involved in determining where and how synapses are formed.
Other studies found that this transcription factor was involved in synaptic strength. In this study, it was found that the u''nc-4'' pathway negatively regulates ''ceh-12'', a gene involved in regulating synaptic choice.
Growth cones and guidance cues
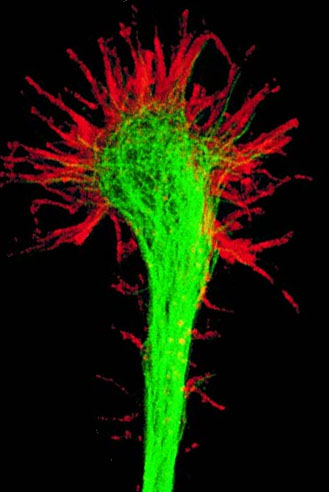
Guidance cues are essential for nervous system development as well as synaptic maintenance and remodeling.
Guidance cues--attractive or repulsive--are sensed by growth cones. Expression of guidance cue genes is mediated at the transcriptional, post-transcriptional, translational, and post-translational levels.
Most guidance cues converge onto various families of small GTPases which go back and forth from active to inactive forms. There are a multitude of signaling pathways involved in this process but the key ones involve netrins (NTNs) and fibronectin leucine-rich repeat transmembrane proteins (FLRTs), the ''Slit'' family, semamorphins (SEMA),
ephrin
Ephrins (also known as ephrin ligands or Eph family receptor interacting proteins) are a family of proteins that serve as the ligands of the Eph receptor. Eph receptors in turn compose the largest known subfamily of receptor protein-tyrosine ki ...
, non-canonical genes (morphogens, chemokines, growth factors), and RTN4 receptors.
= Netrin and FLRTs signaling pathways
=
NTNs and FLRTs both act as guidance cues. NTNs may act as attractants or repellents by DCC and neogenin1, or repellants by UNC5 receptors. UNC5s also act as repulsive receptors for FLRTs. Besides guidance cues, NTNs and FLRTs are also involved in synaptic specificity and synaptogenesis.
In studying
Netrin, one study found that Netrin is not needed for long-range guidance decision, but is used for short-range synaptic targeting. This was determined from studying an RP3 axon, which expresses Netrin as an axonal guidance cue. In gene knockout studies of Netrin, the RP3 growth cone still formed the correct synapses but the connections were not strong.
Elimination Mechanisms of Transient Projections
In exuberant synaptogenesis, many of the projections formed are eliminated either by neuronal death or selective deletion.
By using
retrograde tracing
Retrograde tracing is a research method used in neuroscience to trace neural connections from their point of termination (the synapse) to their source (the cell body). Retrograde tracing techniques allow for detailed assessment of neuronal connec ...
to label transient projections, researchers were able to detect the mechanism of selection axonal deletion. Most of the evidence is provided from studying axonal elimination in the visual cortex, so more research is necessary. However, current research proposes that this elimination mechanism involves retraction of branches over short distances in addition to degeneration of long branches.
The main question that researchers are asking is: what triggers axonal elimination of exuberant synapses? In one study, researchers determined that mice mutant for
semaphorin
Semaphorins are a class of secreted and membrane proteins that were originally identified as axonal growth cone guidance molecules. They primarily act as short-range inhibitory signals and signal through multimeric receptor (biochemistry), recepto ...
, a molecule that is chemorepulsive to growth cones, had defective pruning in hippocampal
mossy fibers. Other chemorepulsive molecules include Slits and ephrins.
Synaptic adhesion molecules (SAMs)
Synaptic adhesion molecules (SAMs) have been presented by researchers as potentially key molecules involved in the organization of synaptic junctions. SAMs are involved in pre- to postsynaptic signaling and the reverse direction.
Distribution
SAMs often form heterophilic complexes that differ based on location. For example, presynaptic SAMs are present on excitatory and inhibitory synapses. In comparison, post synaptic SAMs are very diverse and are specific for excitatory or inhibitory synapses.
Classification
The most well-studied SAMs involved in developing and mature synapses include neurexins and neuroligins, EphBs and ephrin-Bs, immunoglobulin (Ig)-containing cell adhesion molecules and cadherins.
Neurexins and neuroligins
Studies demonstrate that both neurexins and neuroligins are involved in excitatory and inhibitory synapse formation. Neurexin-neuroligin interactions are also involved in the organization of pre- and postsynaptic terminal components.
There are various subtypes of neurexins and neuroligins which determine their involvement in either excitatory or inhibitory synapse formation. α- and β-neurexin have similar intracellular domains but different sized extracellular domains. Neuroligins bind to neurexins. Neuroligin 1 is involved in excitatory specializations formation, but it depends on the results of
alternative splicing
Alternative splicing, alternative RNA splicing, or differential splicing, is an alternative RNA splicing, splicing process during gene expression that allows a single gene to produce different splice variants. For example, some exons of a gene ma ...
. Neuroligin 2 is localized to inhibitory synapses. Neuroligin 3 is likely involved in excitatory synaptogenesis, but more research needs to be conducted on this.
However, one study found that knockdown of all neuroligins leads to a decrease in frequency of inhibitory but not excitatory miniature synaptic currents.
Both neurexin and neuroligins have a PDZ binding domain that determines what synaptic scaffolding proteins they interact with.
Another important role of neuroligins and neurexins is the determination of where a synapse forms. For example, co-clustering of neuroligin 1 to PSD-95 acts as a hotspot for presynaptic machinery.
EphBs and Ephrin-Bs
Ephs can be divided into A and B subclasses based on affinity for ephrin-A or ephrin-B ligands. Studies reveal that mainly ''EphB-ephrin-B'' interactions are involved in synaptogenesis.
The binding of EphB to Ephrin-B leads to bidirectional signaling and contact-mediated transcellular signaling. During development, this interaction is primarily involved in axon guidance and boundary formation. However, these signaling molecules have also been shown to modify postsynaptic organization.
EphBs are particularly involved in excitatory synaptogenesis. When activated by soluble ephrin-B-Fc fusion protein, EphB induces clustering of NMDARs and AMPARs, an increase in the number of presynaptic terminals, and the formation of dendritic spines. Lastly, binding of Ephrin-B to EphB2 leads to interactions between the extracellular domains of the NMDAR and EphB2.
Immunoglobulins
A key characteristic of Ig molecules is the diverse number of globular extracellular cysteine-looped domains. A number of members of the Ig superfamily have been identified as essential molecules for the organization of pre and post synaptic domains. These include synaptic cell adhesion molecules (SynCAM), synaptic adhesion-like molecules (SALMs), netrin G2 ligand (NGL2), neural cell adhesion molecule (NCAM), etc.
Cadherins
Neuronal (N)-cadherins are found in pre and postsynaptic terminals. Prior to differentiation, N-cadherins increase in quantity at axon-dendrite contact sites and eventually restrict their presence to sites around the active zone in mature neurons. N-cadherin is also involved in regulating AMPAR trafficking.
Besides this, N-cadherin also plays a role in the maturation and stabilization of synaptic specializations. Lastly, N-cadherins help to control dendritic spine morphology and motility.
Function
The main function of SAMs in a broad sense includes forming the synapse and determining the properties of synapse.
Synaptic specificity
In general, three processes are involved in determining the locations and properties of synapses. To determine location, axon guidance is coupled to partner choice which are processes both guided by SAMs. However, this process is still unclear. Previous studies demonstrate that axon guidance involves non-synaptic adhesion molecules. Researchers hypothesize that partner choice is initiated by SAMs.
The mechanisms by which partner choice is determined is also not clear. However, three hypotheses have been proposed to help explain how synapse specificity is determined:
# Partner is choice is determined and then synapse formation occurs, implying they are separate processes mechanistically
# Partner choice and synapse formation are the same process and both are determined by SAMs
# Synapse formation occurs and then a selective elimination process.
However, studies observing a heterologous synapse formation assay and the involvement of SAM in non neuronal cells indicates that hypothesis 1 and 2 are most likely.
Currently, the only SAMs known to be involved in establishing proteins are: postsynaptic adhesion-GPCRs called latrophilins and brain angiogenesis inhibitors. Similarly, teneurins have been presented as mediators in synapse formation.
Properties of Synapses
The properties of synapses is likely shaped by bidirectional signaling between pre- and postsynaptic specialization and are mediated partly by SAMS. This is demonstrated by studies of
neurexins, the most common type of SAMs.
Recent studies demonstrate that neurexins are necessary for organizing functional synapses and perform important functions depending on the type of neuron. This is generated by different neurexin
isoforms
A protein isoform, or "protein variant", is a member of a set of highly similar proteins that originate from a single gene and are the result of genetic differences. While many perform the same or similar biological roles, some isoforms have uniqu ...
. One example is the difference in function between presynaptic neurexin-1 containing an insert in SS4 (Nrxn1−SS4+) and neurexin-1 lacking an insert in SS4 (Nrxn1−SS4+) generated by alternative splicing. Nrxn1−SS4+ is involved in the trans-synaptic increase in postsynaptic NMDAR levels.
Other SAMs have a similar diversity in function. For example, LAR-PTPRs are also involved in NMDAR-mediated synapse responses. However, the main difference between LAR-PTPRs and neurexin-1 is that in neurexin-1 mediated signaling, surface levels of NMDARs are changed.
Formation of the neuromuscular junction
The
neuromuscular junction
A neuromuscular junction (or myoneural junction) is a chemical synapse between a motor neuron and a muscle fiber.
It allows the motor neuron to transmit a signal to the muscle fiber, causing muscle contraction.
Muscles require innervation to ...
(NMJ) is the most well-characterized synapse in that it provides a simple and accessible structure that allows for easy manipulation and observation. Therefore, the synapse is well-researched due to its size and accessibility in the nervous system.
Function

The synapse itself is composed of three cells: the
motor neuron
A motor neuron (or motoneuron), also known as efferent neuron is a neuron whose cell body is located in the motor cortex, brainstem or the spinal cord, and whose axon (fiber) projects to the spinal cord or outside of the spinal cord to directly o ...
, the
myofiber, and the
Schwann cell
Schwann cells or neurolemmocytes (named after German physiologist Theodor Schwann) are the principal glia of the peripheral nervous system (PNS). Glial cells function to support neurons and in the PNS, also include Satellite glial cell, satellite ...
. In a normally functioning synapse, a signal will cause the motor neuron to depolarize, by releasing the neurotransmitter
acetylcholine
Acetylcholine (ACh) is an organic compound that functions in the brain and body of many types of animals (including humans) as a neurotransmitter. Its name is derived from its chemical structure: it is an ester of acetic acid and choline. Par ...
(ACh). Acetylcholine travels across the synaptic cleft where it reaches acetylcholine receptors (AChR) on the
plasma membrane
The cell membrane (also known as the plasma membrane or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of a cell from the outside environment (the extr ...
of the myofiber, the
sarcolemma
The sarcolemma (''sarco'' (from ''sarx'') from Greek; flesh, and ''lemma'' from Greek; sheath), also called the myolemma, is the cell membrane surrounding a skeletal muscle fibre or a cardiomyocyte.
It consists of a lipid bilayer and a thin ...
. As the AChRs open
ion channel
Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by Gating (electrophysiol ...
s, the membrane depolarizes, causing muscle contraction. The entire synapse is covered in a
myelin sheath
Myelin Sheath ( ) is a lipid-rich material that in most vertebrates surrounds the axons of neurons to insulate them and increase the rate at which electrical impulses (called action potentials) pass along the axon. The myelinated axon can be lik ...
provided by the Schwann cell to insulate and encapsulate the junction.
Another important part of the neuromuscular system and central nervous system are the
astrocyte
Astrocytes (from Ancient Greek , , "star" and , , "cavity", "cell"), also known collectively as astroglia, are characteristic star-shaped glial cells in the brain and spinal cord. They perform many functions, including biochemical control of en ...
s. While originally they were thought to have only functioned as support for the neurons, they play an important role in functional plasticity of synapses.
Origin and movement of cells
During development, each of the three germ layer cell types arises from different regions of the growing embryo. The individual myoblasts originate in the
mesoderm
The mesoderm is the middle layer of the three germ layers that develops during gastrulation in the very early development of the embryo of most animals. The outer layer is the ectoderm, and the inner layer is the endoderm.Langman's Medical ...
and fuse to form a multi-nucleated myotube. During or shortly after myotube formation, motoneurons from the neural tube form preliminary contacts with the myotube. The Schwann cells arise from the neural crest and are led by the axons to their destination. Upon reaching it, they form a loose, unmyelinated covering over the innervating axons. The movement of the axons (and subsequently the Schwann cells) is guided by the growth cone, a filamentous projection of the axon that actively searches for neurotrophins released by the myotube.
The specific patterning of synapse development at the neuromuscular junction shows that the majority of muscles are innervated at their midpoints. Although it may seem that the axons specifically target the midpoint of the myotube, several factors reveal that this is not a valid claim. It appears that after the initial axonal contact, the newly formed myotube proceeds to grow symmetrically from that point of innervation. Coupled with the fact that AChR density is the result of axonal contact instead of the cause, the structural patterns of muscle fibers can be attributed to both myotatic growth as well as axonal innervation.
The preliminary contact formed between the motor neuron and the myotube generates synaptic transmission almost immediately, but the signal produced is very weak. There is evidence that Schwann cells may facilitate these preliminary signals by increasing the amount of spontaneous neurotransmitter release through small molecule signals. After about a week, a fully functional synapse is formed following several types of differentiation in both the post-synaptic muscle cell and the pre-synaptic motor neuron. This pioneer axon is of crucial importance because the new axons that follow have a high propensity for forming contacts with well-established synapses.
Post-synaptic differentiation
The most noticeable difference in the myotube following contact with the motor neuron is the increased concentration of AChR in the plasma membrane of the myotube in the synapse. This increased amount of AChR allows for more effective transmission of synaptic signals, which in turn leads to a more-developed synapse. The density of AChR is > 10,000/μm
2 and approximately 10/μm
2 around the edge. This high concentration of AChR in the synapse is achieved through clustering of AChR, up-regulation of the AChR gene transcription in the post-synaptic nuclei, and down-regulation of the AChR gene in the non-synaptic nuclei.
The signals that initiate post-synaptic differentiation may be neurotransmitters released directly from the axon to the myotube, or they may arise from changes activated in the extracellular matrix of the synaptic cleft.
Clustering
AChR experiences multimerization within the post-synaptic membrane largely due to the signaling molecule
Agrin
Agrin is a large proteoglycan whose best-characterised role is in the development of the neuromuscular junction during embryogenesis. Agrin is named based on its involvement in the aggregation of acetylcholine receptors during synaptogenesi ...
. The axon of the motor neuron releases agrin, a proteoglycan that initiates a cascade that eventually leads to AChR association. Agrin binds to a muscle-specific kinase (
MuSK
Musk is a class of aromatic substances commonly used as base notes in perfumery. They include glandular secretions from animals such as the musk deer, numerous plants emitting similar fragrances, and artificial substances with similar odors. ' ...
) receptor in the post-synaptic membrane, and this in turn leads to downstream activation of the cytoplasmic protein
Rapsyn. Rapsyn contains domains that allow for AChR association and multimerization, and it is directly responsible for AChR clustering in the post-synaptic membrane: rapsyn-deficient mutant mice fail to form AChR clusters.
Synapse-specific transcription
The increased concentration of AChR is not simply due to a rearrangement of pre-existing synaptic components. The axon also provides signals that regulate gene expression within the myonuclei directly beneath the synapse. This signaling provides for localized up-regulation of transcription of AChR genes and consequent increase in local AChR concentration. The two signaling molecules released by the axon are
calcitonin gene-related peptide
Calcitonin gene-related peptide (CGRP) is a neuropeptide that belongs to the calcitonin family. Human CGRP consists of two Protein isoform, isoforms, CGRP alpha (α-CGRP, also known as CGRP I) and CGRP beta (β-CGRP, also known as CGRP II). α-C ...
(CGRP) and
neuregulin
Neuregulins are a family of four structurally related proteins that are part of the epidermal growth factor family, EGF family of proteins. These proteins have been shown to have diverse functions in the development of the nervous system and play ...
, which trigger a series of kinases that eventually lead to transcriptional activation of the AChR genes.
Extrasynaptic repression
Repression of the AChR gene in the non-synaptic nuclei is an activity-dependent process involving the electrical signal generated by the newly formed synapse. Reduced concentration of AChR in the extrasynaptic membrane in addition to increased concentration in the post-synaptic membrane helps ensure the fidelity of signals sent by the axon by localizing AChR to the synapse. Because the synapse begins receiving inputs almost immediately after the motoneuron comes into contact with the myotube, the axon quickly generates an action potential and releases ACh. The depolarization caused by AChR induces muscle contraction and simultaneously initiates repression of AChR gene transcription across the entire muscle membrane. Note that this affects gene transcription at a distance: the receptors that are embedded within the post-synaptic membrane are not susceptible to repression.
Pre-synaptic differentiation
Although the mechanisms regulating pre-synaptic differentiation are unknown, the changes exhibited at the developing axon terminal are well characterized. The pre-synaptic axon shows an increase in synaptic volume and area, an increase of synaptic vesicles, clustering of vesicles at the active zone, and polarization of the pre-synaptic membrane. These changes are thought to be mediated by neurotrophin and cell adhesion molecule release from muscle cells, thereby emphasizing the importance of communication between the motoneuron and the myotube during synaptogenesis. Like post-synaptic differentiation, pre-synaptic differentiation is thought to be due to a combination of changes in gene expression and a redistribution of pre-existing synaptic components. Evidence for this can be seen in the up-regulation of genes expressing vesicle proteins shortly after synapse formation as well as their localization at the synaptic terminal.
Synaptic maturation
Immature synapses are innervated at birth, due to the high propensity for new axons to innervate at a pre-existing synapse. As the synapse matures, the synapses segregate and eventually all axonal inputs except for one retract in a process called synapse elimination. Furthermore, the post-synaptic end plate grows deeper and creates folds through invagination to increase the surface area available for neurotransmitter reception. At birth, Schwann cells form loose, unmyelinated covers over groups of synapses, but as the synapse matures, Schwann cells become dedicated to a single synapse and form a myelinated cap over the entire neuromuscular junction.
Synapse elimination
The process of synaptic pruning known as synapse elimination is a presumably activity-dependent process that involves competition between axons. Hypothetically, a synapse strong enough to produce an action potential will trigger the myonuclei directly across from the axon to release synaptotrophins that will strengthen and maintain well-established synapses. This synaptic strengthening is not conferred upon the weaker synapses, thereby starving them out. It has also been suggested that in addition to the synaptotrophins released to the synapse exhibiting strong activity, the depolarization of the post-synaptic membrane causes release of synaptotoxins that ward off weaker axons.
Synapse formation specificity
A remarkable aspect of synaptogenesis is the fact that motor neurons are able to distinguish between fast and slow-twitch muscle fibers; fast-twitch muscle fibers are innervated by "fast" motor neurons, and slow-twitch muscle fibers are innervated by "slow" motor neurons. There are two hypothesized paths by which the axons of motor neurons achieve this specificity, one in which the axons actively recognize the muscles that they innervate and make selective decisions based on inputs, and another that calls for more indeterminate innervation of muscle fibers. In the selective paths, the axons recognize the fiber type, either by factors or signals released specifically by the fast or slow-twitch muscle fibers. In addition, selectivity can be traced to the lateral position that the axons are predeterminately arranged in order to link them to the muscle fiber that they will eventually innervate. The hypothesized non-selective pathways indicate that the axons are guided to their destinations by the matrix through which they travel. Essentially, a path is laid out for the axon and the axon itself is not involved in the decision-making process. Finally, the axons may non-specifically innervate muscle fibers and cause the muscles to acquire the characteristics of the axon that innervates them. In this path, a "fast" motoneuron can convert any muscle fiber into a fast-twitch muscle fiber. There is evidence for both selective and non-selective paths in synapse formation specificity, leading to the conclusion that the process is a combination of several factors.
Synapse formation
Although the study of synaptogenesis within the central nervous system (CNS) is much more recent than that of the NMJ, there is promise of relating the information learned at the NMJ to synapses within the CNS. Many similar structures and basic functions exist between the two types of neuronal connections. At the most basic level, the CNS synapse and the NMJ both have a nerve terminal that is separated from the postsynaptic membrane by a cleft containing specialized extracellular material. Both structures exhibit localized vesicles at the active sites, clustered receptors at the post-synaptic membrane, and glial cells that encapsulate the entire synaptic cleft. In terms of synaptogenesis, both synapses exhibit differentiation of the pre- and post-synaptic membranes following initial contact between the two cells. This includes the clustering of receptors, localized up-regulation of protein synthesis at the active sites, and neuronal pruning through synapse elimination.
Despite these similarities in structure, there is a fundamental difference between the two connections. The CNS synapse is strictly neuronal and does not involve muscle fibers: for this reason the CNS uses different neurotransmitter molecules and receptors. More importantly, neurons within the CNS often receive multiple inputs that must be processed and integrated for successful transfer of information. Muscle fibers are innervated by a single input and operate in an all or none fashion. Coupled with the plasticity that is characteristic of the CNS neuronal connections, it is easy to see how increasingly complex CNS circuits can become.
Regulation
Signaling
The main method of synaptic signaling in the NMJ is through use of the neurotransmitter acetylcholine and its receptor. The CNS homolog is glutamate and its receptors, and one of special significance is the N-methyl-D-aspartate (NMDA) receptor. It has been shown that activation of NMDA receptors initiates synaptogenesis through activation of downstream products. The heightened level of NMDA receptor activity during development allows for increased influx of calcium, which acts as a secondary signal. Eventually,
immediate early genes are activated by transcription factors and the proteins required for neuronal differentiation are translated. The NMDA receptor function is associated with the estrogen receptor in hippocampal neurons. Experiments conducted with estradiol show that exposure to the estrogen significantly increases synaptic density and protein concentration.
Synaptic signaling during synaptogenesis is not only activity-dependent, but is also dependent on the environment in which the neurons are located. For instance,
brain-derived neurotrophic factor
Brain-derived neurotrophic factor (BDNF), or abrineurin, is a protein found in the and the periphery. that, in humans, is encoded by the ''BDNF'' gene. BDNF is a member of the neurotrophin family of growth factors, which are related to the can ...
(BDNF) is produced by the brain and regulates several functions within the developing synapse, including enhancement of transmitter release, increased concentration of vesicles, and cholesterol biosynthesis. Cholesterol is essential to synaptogenesis because the lipid rafts that it forms provide a scaffold upon which numerous signaling interactions can occur. BDNF-null mutants show significant defects in neuronal growth and synapse formation. Aside from neurotrophins, cell-adhesion molecules are also essential to synaptogenesis. Often the binding of pre-synaptic cell-adhesion molecules with their post-synaptic partners triggers specializations that facilitate synaptogenesis. Indeed, a defect in genes encoding
neuroligin, a cell-adhesion molecule found in the post-synaptic membrane, has been linked to cases of
autism
Autism, also known as autism spectrum disorder (ASD), is a neurodevelopmental disorder characterized by differences or difficulties in social communication and interaction, a preference for predictability and routine, sensory processing d ...
and mental retardation. Finally, many of these signaling processes can be regulated by
matrix metalloproteinases
Matrix metalloproteinases (MMPs), also known as matrix metallopeptidases or matrixins, are metalloproteinases that are calcium-dependent zinc-containing endopeptidases; other family members are adamalysins, serralysins, and astacins. The MMPs be ...
(MMPs) as the targets of many MMPs are these specific cell-adhesion molecules.
[
]
Morphology
The special structure found in the CNS that allows for multiple inputs is the dendritic spine
A dendritic spine (or spine) is a small membrane protrusion from a neuron's dendrite that typically receives input from a single axon at the synapse. Dendritic spines serve as a storage site for synaptic strength and help transmit electrical sign ...
, the highly dynamic site of excitatory synapses. This morphological dynamism is due to the specific regulation of the actin cytoskeleton, which in turn allows for regulation of synapse formation. Dendritic spines exhibit three main morphologies: filopodia, thin spines, and mushroom spines. The filopodia play a role in synaptogenesis through initiation of contact with axons of other neurons. Filopodia of new neurons tend to associate with multiply synapsed axons, while the filopodia of mature neurons tend to sites devoid of other partners. The dynamism of spines allows for the conversion of filopodia into the mushroom spines that are the primary sites of glutamate receptors and synaptic transmission.
Contributions of the Wnt protein family
The ( Wnt) family, includes several embryonic morphogen
A morphogen is a substance whose non-uniform distribution governs the pattern of tissue development in the process of morphogenesis or pattern formation, one of the core processes of developmental biology, establishing positions of the various ...
s that contribute to early pattern formation in the developing embryo. Recently data have emerged showing that the Wnt protein family has roles in the later development of synapse formation and plasticity. Wnt contribution to synaptogenesis has been verified in both the central nervous system
The central nervous system (CNS) is the part of the nervous system consisting primarily of the brain, spinal cord and retina. The CNS is so named because the brain integrates the received information and coordinates and influences the activity o ...
and the neuromuscular junction
A neuromuscular junction (or myoneural junction) is a chemical synapse between a motor neuron and a muscle fiber.
It allows the motor neuron to transmit a signal to the muscle fiber, causing muscle contraction.
Muscles require innervation to ...
.
Central nervous system
Wnt family members contribute to synapse formation in the cerebellum
The cerebellum (: cerebella or cerebellums; Latin for 'little brain') is a major feature of the hindbrain of all vertebrates. Although usually smaller than the cerebrum, in some animals such as the mormyrid fishes it may be as large as it or eve ...
by inducing presynaptic
In the nervous system, a synapse is a structure that allows a neuron (or nerve cell) to pass an electrical or chemical signal to another neuron or a target effector cell. Synapses can be classified as either chemical or electrical, depending o ...
and postsynaptic terminal formation. This brain region contains three main neuronal cell types- Purkinje cells, granule cells and mossy fiber cells. Wnt-3 expression contributes to Purkinje cell neurite outgrowth and synapse formation.growth cone
A growth cone is a large actin-supported extension of a developing or regenerating neurite seeking its synaptic target. It is the growth cone that drives axon growth. Their existence was originally proposed by Spanish histologist Santiago ...
enlargement by spreading microtubules
Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to eukaryotic cells. Microtubules can be as long as 50 micrometres, as wide as 23 to 27 nm and have an inner diameter between 11 an ...
.synaptic vesicles
In a neuron, synaptic vesicles (or neurotransmitter vesicles) store various neurotransmitters that are released at the synapse. The release is regulated by a voltage-dependent calcium channel. Vesicles are essential for propagating nerve impul ...
and presynaptic proteins to the synaptic active zone.PSD-95
PSD-95 (postsynaptic density protein 95) also known as SAP-90 (synapse-associated protein 90) is a protein that in humans is encoded by the ''DLG4'' (discs large homolog 4) gene.
PSD-95 is a member of the membrane-associated guanylate kinase (MA ...
.hippocampus
The hippocampus (: hippocampi; via Latin from Ancient Greek, Greek , 'seahorse'), also hippocampus proper, is a major component of the brain of humans and many other vertebrates. In the human brain the hippocampus, the dentate gyrus, and the ...
Wnts in conjunction with cell electrical activity promote synapse formation. Wnt7b is expressed in maturing dendrites,Frizzled
Frizzled is a family of atypical G protein-coupled receptor, G protein-coupled receptors that serve as receptors in the Wnt signaling pathway and other signaling pathways. When activated, Frizzled leads to activation of Dishevelled in the cytosol ...
(Fz), increases highly with synapse formation in the hippocampus.glutamate
Glutamic acid (symbol Glu or E; known as glutamate in its anionic form) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a Essential amino acid, non-essential nutrient for humans, meaning that ...
receptor activation increases Wnt2 expression. Long term potentiation (LTP) due to NMDA activation and subsequent Wnt expression leads to Fz-5 localization at the postsynaptic active zone.
Neuromuscular junction
Similar mechanisms of action by Wnts in the central nervous system are observed in the neuromuscular junction (NMJ) as well. In the Drosophila
''Drosophila'' (), from Ancient Greek δρόσος (''drósos''), meaning "dew", and φίλος (''phílos''), meaning "loving", is a genus of fly, belonging to the family Drosophilidae, whose members are often called "small fruit flies" or p ...
NMJ mutations in the Wnt5 receptor Derailed (drl) reduce the number of and density of synaptic active zones.neurotransmitter
A neurotransmitter is a signaling molecule secreted by a neuron to affect another cell across a Chemical synapse, synapse. The cell receiving the signal, or target cell, may be another neuron, but could also be a gland or muscle cell.
Neurotra ...
in this system is glutamate. Wnt is needed to localize glutamatergic
Glutamatergic means "related to glutamate". A glutamatergic agent (or drug) is a chemical that directly modulates the excitatory amino acid (glutamate/aspartate) system in the body or brain. Examples include excitatory amino acid receptor agonist ...
receptors on postsynaptic muscle cells. As a result, Wnt mutations diminish evoked currents on the postsynaptic muscle.acetylcholine receptor
An acetylcholine receptor (abbreviated AChR) or a cholinergic receptor is an integral membrane protein that responds to the binding of acetylcholine, a neurotransmitter.
Classification
Like other transmembrane receptors, acetylcholine receptor ...
(AChR) clustering in the postsynaptic density of muscle cells. Wnt-3 is expressed by muscle fibers and is secreted retrogradely onto motor neurons.Agrin
Agrin is a large proteoglycan whose best-characterised role is in the development of the neuromuscular junction during embryogenesis. Agrin is named based on its involvement in the aggregation of acetylcholine receptors during synaptogenesi ...
to promote growth cone enlargement, axon branching and synaptic vesicle clustering.
Synaptogenesis in the Mature Brain
Although synaptogenesis occurs more commonly in the developing brain, imaging reveals that approximately 40% of dendritic spines found in the sensory and motor cortices are replaced every 5 days.olfactory bulb
The olfactory bulb (Latin: ''bulbus olfactorius'') is a neural structure of the vertebrate forebrain involved in olfaction, the sense of smell. It sends olfactory information to be further processed in the amygdala, the orbitofrontal cortex (OF ...
(OB) as well as the dentate gyrus
The dentate gyrus (DG) is one of the subfields of the hippocampus, in the hippocampal formation. The hippocampal formation is located in the temporal lobe of the brain, and includes the hippocampus (including CA1 to CA4) subfields, and other su ...
(DG) of the hippocampus
The hippocampus (: hippocampi; via Latin from Ancient Greek, Greek , 'seahorse'), also hippocampus proper, is a major component of the brain of humans and many other vertebrates. In the human brain the hippocampus, the dentate gyrus, and the ...
. The three main types of neurons that are added during this process include granule cells
The name granule cell has been used for a number of different types of neurons whose only common feature is that they all have very small cell bodies. Granule cells are found within the granular layer of the cerebellum, the dentate gyrus of t ...
and periglomerular neurons (PGNs) in the OB and granule cells in the DG. However, granule cells in the OB are the largest population of new neurons formed in the adult brain.
Adult synaptogenesis in the dentate gyrus
Granule cells formed during the adult period In the dentate gyrus are excitatory neurons that receive glutamate from projection neurons in the entorhinal cortex and mossy cells in the hippocampus, as well as GABA from local interneurons. These neurons have projections to the CA3 region of the hippocampus.
Adult synaptogenesis in the olfactory bulb
Periglomerular neurons
PGNs are classified as GABAergic or dopaminergic modulating interneurons. These receive input from olfactory sensory neurons which project to the dendrites of the primary neurons of the OB. These neurons surround glomeruli that contain olfactory sensory axons that connect to the primary neurons of the OB.
Unfortunately, not much is known about the development of these neurons in the adult brain. However, it was revealed by two-photon imaging that as these neurons mature, the dendritic spines become more stable. Additionally, studies reveal that at the postsynaptic sites of PGNs there are functional changes between sensory neurons and PGNs. For example, at olfactory nerve (ON) synapses there is an increase in the AMPA:NMDA ratio as the brain matures.
Granule neurons
Granule neurons of the OB are axonless GABAergic interneurons which connect to the primary neurons of the OB.
References
{{reflist, 2
Developmental neuroscience
 Guidance cues are essential for nervous system development as well as synaptic maintenance and remodeling. Guidance cues--attractive or repulsive--are sensed by growth cones. Expression of guidance cue genes is mediated at the transcriptional, post-transcriptional, translational, and post-translational levels.
Most guidance cues converge onto various families of small GTPases which go back and forth from active to inactive forms. There are a multitude of signaling pathways involved in this process but the key ones involve netrins (NTNs) and fibronectin leucine-rich repeat transmembrane proteins (FLRTs), the ''Slit'' family, semamorphins (SEMA),
Guidance cues are essential for nervous system development as well as synaptic maintenance and remodeling. Guidance cues--attractive or repulsive--are sensed by growth cones. Expression of guidance cue genes is mediated at the transcriptional, post-transcriptional, translational, and post-translational levels.
Most guidance cues converge onto various families of small GTPases which go back and forth from active to inactive forms. There are a multitude of signaling pathways involved in this process but the key ones involve netrins (NTNs) and fibronectin leucine-rich repeat transmembrane proteins (FLRTs), the ''Slit'' family, semamorphins (SEMA),