Supersecondary structure on:
[Wikipedia]
[Google]
[Amazon]
A supersecondary structure is a compact three-dimensional


 A Greek key motif has four features:
# Four sequentially connected beta strands are adjacent to, but not necessarily geometrically aligned with, each other.
# The beta sheet is anti-parallel, and alternate strands run in the same directions.
# The first strand and last strand are next to each other and bonded by hydrogen bonds.
# Connecting loops can be long and include other secondary structures.
The Greek key motif has its name because the structure looks like the pattern seen on Greek urns. This motif has no known function.
A Greek key motif has four features:
# Four sequentially connected beta strands are adjacent to, but not necessarily geometrically aligned with, each other.
# The beta sheet is anti-parallel, and alternate strands run in the same directions.
# The first strand and last strand are next to each other and bonded by hydrogen bonds.
# Connecting loops can be long and include other secondary structures.
The Greek key motif has its name because the structure looks like the pattern seen on Greek urns. This motif has no known function.
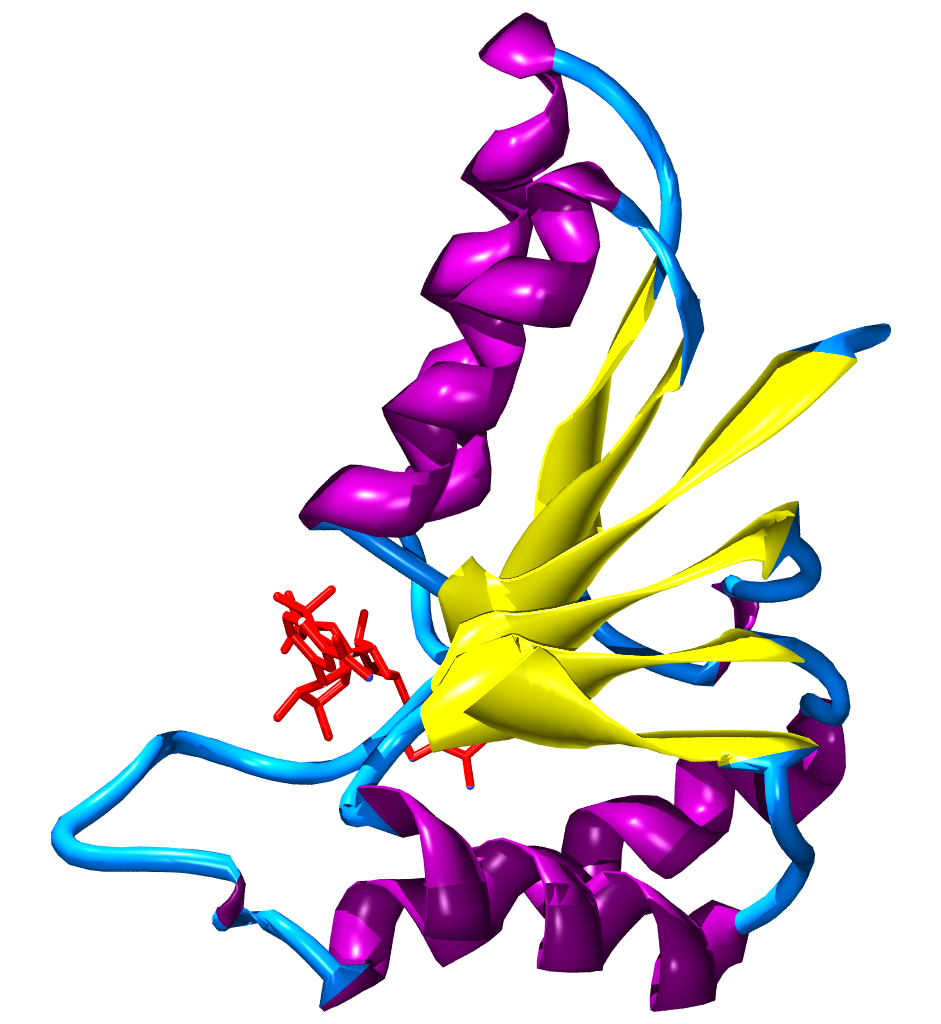 A beta-alpha-beta motif is composed of two beta strands joined by an alpha helix through connecting loops. The beta strands are parallel, and the helix is also almost parallel to the strands. This structure can be seen in almost all proteins with parallel strands. The loops connecting the beta strands and alpha helix can vary in length and often binds ligands.
Beta-alpha-beta helices can be either left-handed or right-handed. When viewed from the N-terminal side of the beta strands, so that one strand is on top of the other, a left-handed beta-alpha-beta motif has the alpha helix on the left side of the beta strands. The more common right-handed motif would have an alpha helix on the right side of the plane containing the beta strands.
A beta-alpha-beta motif is composed of two beta strands joined by an alpha helix through connecting loops. The beta strands are parallel, and the helix is also almost parallel to the strands. This structure can be seen in almost all proteins with parallel strands. The loops connecting the beta strands and alpha helix can vary in length and often binds ligands.
Beta-alpha-beta helices can be either left-handed or right-handed. When viewed from the N-terminal side of the beta strands, so that one strand is on top of the other, a left-handed beta-alpha-beta motif has the alpha helix on the left side of the beta strands. The more common right-handed motif would have an alpha helix on the right side of the plane containing the beta strands.
protein structure
Protein structure is the three-dimensional arrangement of atoms in an amino acid-chain molecule. Proteins are polymers specifically polypeptides formed from sequences of amino acids, the monomers of the polymer. A single amino acid monomer ma ...
of several adjacent elements of a secondary structure
Protein secondary structure is the three dimensional conformational isomerism, form of ''local segments'' of proteins. The two most common Protein structure#Secondary structure, secondary structural elements are alpha helix, alpha helices and beta ...
that is smaller than a protein domain
In molecular biology, a protein domain is a region of a protein's polypeptide chain that is self-stabilizing and that folds independently from the rest. Each domain forms a compact folded three-dimensional structure. Many proteins consist of s ...
or a subunit. Supersecondary structures can act as nucleation
In thermodynamics, nucleation is the first step in the formation of either a new thermodynamic phase or structure via self-assembly or self-organization within a substance or mixture. Nucleation is typically defined to be the process that deter ...
s in the process of protein folding
Protein folding is the physical process by which a protein chain is translated to its native three-dimensional structure, typically a "folded" conformation by which the protein becomes biologically functional. Via an expeditious and reproduci ...
.
Examples
Helix supersecondary structures

Helix hairpin
A helix hairpin, also known as an alpha-alpha hairpin, is composed of two antiparallelalpha helices
The alpha helix (α-helix) is a common motif in the secondary structure of proteins and is a right hand-helix conformation in which every backbone N−H group hydrogen bonds to the backbone C=O group of the amino acid located four residues ear ...
connected by a loop of two or more residues. True to its name, it resembles a hairpin. A longer loop has a greater number of possible conformations. If short strands connect the helices, then the individual helices will pack together through their hydrophobic residues. The function of a helix hairpin is unknown; however, a four helix bundle is composed of two helix hairpins, which have important ligand binding sites.
Helix corner
A helix corner, also called an alpha-alpha corner, has two alpha helices almost at right angles to each other connected by a short 'loop'. This loop is formed from ahydrophobic
In chemistry, hydrophobicity is the physical property of a molecule that is seemingly repelled from a mass of water (known as a hydrophobe). In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, t ...
residue. The function of a helix corner is unknown.
Helix-loop-helix
The helix-loop-helix structure has two helices connected by a 'loop'. These are fairly common and usually bindligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electr ...
s. For example, calcium binds with the carboxyl groups of the side chain
In organic chemistry and biochemistry, a side chain is a chemical group that is attached to a core part of the molecule called the "main chain" or backbone. The side chain is a hydrocarbon branching element of a molecule that is attached to a ...
s within the loop region between the helices.
Helix-turn-helix
The helix-turn-helix motif is important for DNA binding and is therefore in many DNA binding proteins.Beta sheet supersecondary structures

Beta hairpin
A beta hairpin is a common supersecondary motif composed of two anti-parallelbeta strands
Beta (, ; uppercase , lowercase , or cursive ; grc, βῆτα, bē̂ta or ell, βήτα, víta) is the second letter of the Greek alphabet. In the system of Greek numerals, it has a value of 2. In Modern Greek, it represents the voiced labio ...
connected by a loop. The structure resembles a hairpin and is often found in globular proteins.
The loop between the beta strands can range anywhere from 2 to 16 residues. However, most loops contain less than seven residues. Residues in beta hairpins with loops of 2, 3, or 4 residues have distinct conformations. However, a wide range of conformations can be seen in longer loops, which are sometimes referred to as 'random coils'. A beta-meander consists of ''consecutive'' antiparallel-beta strands linked by hairpins.
Two residue loops are called beta turns or reverse turns. Type I' and Type II' reverse turns occur most frequently because they have less steric hindrance than Type I and Type II turns. The function of beta hairpins is unknown.
Beta corner
A beta hairpin has two antiparallel beta strands that are at about a 90 degree angle to each other. It is formed by a beta hairpin changing direction with one strand having aglycine
Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid (carbamic acid is unstable), with the chemical formula NH2‐ CH2‐ COOH. Glycine is one of the proteinogeni ...
residue and the other strand having a beta bulge A beta bulge can be described as a localized disruption of the regular hydrogen bonding of beta sheet by inserting extra residues into one or both hydrogen bonded β-strands.
Types
β-bulges can be grouped according to their length of the disruptio ...
. Beta corners have no known function.
Greek key motif
= Other
=β-sheets
The beta sheet, (β-sheet) (also β-pleated sheet) is a common motif of the regular protein secondary structure. Beta sheets consist of beta strands (β-strands) connected laterally by at least two or three backbone hydrogen bonds, forming a gen ...
(composed of multiple hydrogen-bonded individual β-strands) are sometimes considered a secondary or supersecondary structure.
Mixed supersecondary structures
Beta-alpha-beta motifs
 A beta-alpha-beta motif is composed of two beta strands joined by an alpha helix through connecting loops. The beta strands are parallel, and the helix is also almost parallel to the strands. This structure can be seen in almost all proteins with parallel strands. The loops connecting the beta strands and alpha helix can vary in length and often binds ligands.
Beta-alpha-beta helices can be either left-handed or right-handed. When viewed from the N-terminal side of the beta strands, so that one strand is on top of the other, a left-handed beta-alpha-beta motif has the alpha helix on the left side of the beta strands. The more common right-handed motif would have an alpha helix on the right side of the plane containing the beta strands.
A beta-alpha-beta motif is composed of two beta strands joined by an alpha helix through connecting loops. The beta strands are parallel, and the helix is also almost parallel to the strands. This structure can be seen in almost all proteins with parallel strands. The loops connecting the beta strands and alpha helix can vary in length and often binds ligands.
Beta-alpha-beta helices can be either left-handed or right-handed. When viewed from the N-terminal side of the beta strands, so that one strand is on top of the other, a left-handed beta-alpha-beta motif has the alpha helix on the left side of the beta strands. The more common right-handed motif would have an alpha helix on the right side of the plane containing the beta strands.Rossman fold
Rossman folds, named after Michael Rossman, consist of 3 beta strands and 2 helices in an alternating fashion: beta strand, helix, beta strand, helix, beta strand. This motif tends to reverse the direction of the chain within a protein. Rossman folds have an important biological function in binding nucleotides such as NAD within most dehydrogenases.See also
*Protein folding
Protein folding is the physical process by which a protein chain is translated to its native three-dimensional structure, typically a "folded" conformation by which the protein becomes biologically functional. Via an expeditious and reproduci ...
*Secondary structure
Protein secondary structure is the three dimensional conformational isomerism, form of ''local segments'' of proteins. The two most common Protein structure#Secondary structure, secondary structural elements are alpha helix, alpha helices and beta ...
*Structural motif
In a polymer, chain-like biological molecule, such as a protein or nucleic acid, a structural motif is a common Biomolecular structure#Tertiary structure, three-dimensional structure that appears in a variety of different, evolutionarily unrel ...
References
Further reading
* {{DEFAULTSORT:Supersecondary Structure Protein structural motifs