Sultam MBH on:
[Wikipedia]
[Google]
[Amazon]
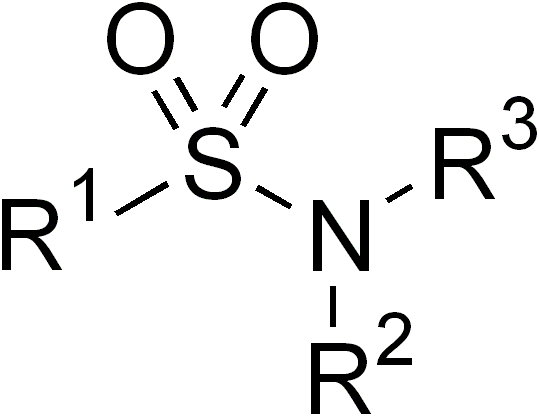 In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the
R^1SO2Cl + R^2R^3NH -> R^1SO2NR^2R^3 + HCl
A base such as pyridine is typically added to absorb the HCl that is generated. Illustrative is the synthesis of sulfonylmethylamide. A readily available sulfonyl chloride source is
File:Saccharin.svg, Saccharin, a cyclic sulfonamide that was one of the first
 In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the structure
A structure is an arrangement and organization of interrelated elements in a material object or system, or the object or system so organized. Material structures include man-made objects such as buildings and machines and natural objects such as ...
. It consists of a sulfonyl group () connected to an amine group (). Relatively speaking this group is unreactive
In chemistry, reactivity is the impulse for which a chemical substance undergoes a chemical reaction, either by itself or with other materials, with an overall release of energy.
''Reactivity'' refers to:
* the chemical reactions of a single sub ...
. Because of the rigidity of the functional group, sulfonamides are typically crystalline; for this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point. Many important drugs contain the sulfonamide group.
A sulfonamide (compound) is a chemical compound that contains this group. The general formula is or , where each R is some organic group; for example, "methanesulfonamide" (where R = methane, R' = R" = hydrogen) is . Any sulfonamide can be considered as derived from a sulfonic acid
In organic chemistry, sulfonic acid (or sulphonic acid) refers to a member of the class of organosulfur compounds with the general formula , where R is an organic alkyl or aryl group and the group a sulfonyl hydroxide. As a substituent, it is kn ...
by replacing a hydroxyl group () with an amine group.
In medicine, the term "sulfonamide" is sometimes used as a synonym for sulfa drug
Sulfonamide is a functional group (a part of a molecule) that is the basis of several groups of drugs, which are called sulphonamides, sulfa drugs or sulpha drugs. The original antibacterial sulfonamides are synthetic (nonantibiotic) antimi ...
, a derivative or variation of sulfanilamide. The first sulfonamide was discovered in Germany in 1932.
Synthesis
Sulfonamides can be prepared in the laboratory in many ways. The classic approach entails the reaction of sulfonyl chlorides with an amine. :tosyl chloride
4-Toluenesulfonyl chloride (''p''-toluenesulfonyl chloride, toluene-''p''-sulfonyl chloride) is an organic compound with the formula CH3C6H4SO2Cl. This white, malodorous solid is a reagent widely used in organic synthesis. Abbreviated TsCl or To ...
. The reaction of primary and secondary amines with benzenesulfonyl chloride is the basis of the Hinsberg reaction, a method for detecting primary and secondary amines.
Sultams
Sultams are cyclic sulfonamides. Bioactive sultams include the antiinflammatory ampiroxicam and the anticonvulsant sulthiame. Sultams are prepared analogously to other sulfonamides, allowing for the fact that sulfonic acids are deprotonated by amines. They are often prepared by one-pot oxidation of disulfides or thiols linked to amines. An alternative synthesis of sultams involves initial preparation of a linear sulfonamide, followed by intramolecular C-C bond formation (i.e. cyclization), a strategy that was used in the synthesis of a sultam-based deep-blue emitter for organic electronics.artificial sweeteners
A sugar substitute is a food additive that provides a sweetness like that of sugar while containing significantly less food energy than sugar-based sweeteners, making it a zero-calorie () or low-calorie sweetener. Artificial sweeteners may be d ...
discovered.
File:Sulfanilamide-skeletal.svg, Sulfanilamide
Sulfanilamide (also spelled sulphanilamide) is a sulfonamide antibacterial drug. Chemically, it is an organic compound consisting of an aniline derivatized with a sulfonamide group. Powdered sulfanilamide was used by the Allies in World War II ...
, a compound that foreshadowed the development of sulfa drugs.
File:Sulfamethoxazole-skeletal.svg, Sulfamethoxazole
Sulfamethoxazole (SMZ or SMX) is an antibiotic. It is used for bacterial infections such as urinary tract infections, bronchitis, and prostatitis and is effective against both gram negative and positive bacteria such as ''Listeria monocytogenes' ...
is a widely used antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention of ...
.
File:Ampiroxicam int.svg, Ampiroxicam is a sultam used as an antiinflammatory drug.
File:Hydrochlorothiazide-2D-skeletal.png, Hydrochlorothiazide is a drug that features both acyclic and cyclic sulfonamide groups.
File:Oppolzer sultam.svg , Camphorsultam
Camphorsultam, also known as bornanesultam, is a crystalline solid primarily used as a chiral auxiliary in the synthesis of other chemicals with a specific desired stereoselectivity. Camphorsultam is commercially available in both enantiomers of i ...
is a sultam used as a chiral auxiliary in organic synthesis.
Sulfinamides
The relatedsulfinamide
Sulfinamide is a functional group in organosulfur chemistry with the structural formula RS(O)NR'2 (where R and R' are organic substituents). This functionality is composed of a sulfur-carbon (S–C) and sulfur-nitrogen (S–N) single bonds, as we ...
s (R(S=O)NHR) are amides of sulfinic acid
Sulfinic acids are oxoacids of sulfur with the structure RSO(OH). In these organosulfur compounds, sulfur is pyramidal.
Structure and properties
Sulfinic acids RSO2H are about 1000x more acidic than the corresponding carboxylic acid RCO2H. Su ...
s (R(S=O)OH) (see sulfinyl
In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl () functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. Exa ...
). Chiral sulfinamides such as tert-butanesulfinamide
''tert''-Butanesulfinamide (also known as 2-methyl-2-propanesulfinamide or Ellman's sulfinamide) is an organosulfur compound and a member of the class of sulfinamides. Both enantiomeric forms are commercially available and are used in asymmetr ...
, p-toluenesulfinamide and 2,4,6-trimethylbenzenesulfinamide are relevant to asymmetric synthesis
Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molecul ...
.
Disulfonimides
Bis(trifluoromethanesulfonyl)aniline
Bis(trifluoromethanesulfonyl)aniline is the organic compound with the formula C6H5N(SO2CF3)2.{{cite journal, title=N-Phenyltrifluoromethanesulfonimide
, last1=Zeller, first1=Wayne E., last2=Schwörer, first2=Ralf, journal=E-EROS Encyclopedia of Re ...
is a source of the triflyl
In organic chemistry, the triflyl group (systematic name: trifluoromethanesulfonyl group) is a functional group with the formula and structure . The triflyl group is often represented by –Tf.
The related triflate group (trifluoromethanesulfo ...
() group.
The disulfonimides are of the type with two sulfonyl groups flanking an amine. As with sulfinamides, this class of compounds is used as catalysts in enantioselective
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical anti ...
synthesis.
See also
*Sulfonamide (medicine)
Sulfonamide is a functional group (a part of a molecule) that is the basis of several groups of drugs, which are called sulphonamides, sulfa drugs or sulpha drugs. The original antibacterial sulfonamides are synthetic (nonantibiotic) antimi ...
* Sulfamic acid
* Sulfamide
Sulfamide (IUPAC name: sulfuric diamide) is an organosulfur compound with the chemical formula and structure . Sulfamide is produced by the reaction of sulfuryl chloride with ammonia.
Sulfamide was first prepared in 1838 by the French chemist ...
References
{{Functional groups Functional groups