
The Streeter–Phelps equation is used in the study of
water pollution
Water pollution (or aquatic pollution) is the contamination of water bodies, usually as a result of human activities, so that it negatively affects its uses. Water bodies include lakes, rivers, oceans, aquifers, reservoirs and groundwater. Water ...
as a
water quality modelling
Water quality modeling involves water quality based data using mathematical simulation techniques. Water quality modeling helps people understand the eminence of water quality issues and models provide evidence for policy makers to make decisions ...
tool. The model describes how
dissolved oxygen
Oxygen saturation (symbol SO2) is a relative measure of the concentration of oxygen that is dissolved or carried in a given medium as a proportion of the maximal concentration that can be dissolved in that medium at the given temperature. It ca ...
(DO) decreases in a river or stream along a certain distance by degradation of
biochemical oxygen demand
Biochemical oxygen demand (BOD) is the amount of dissolved oxygen (DO) needed (i.e. demanded) by aerobic biological organisms to break down organic material present in a given water sample at a certain temperature over a specific time period. T ...
(BOD). The equation was derived by H. W. Streeter, a sanitary engineer, and
Earle B. Phelps, a consultant for the
U.S. Public Health Service
The United States Public Health Service (USPHS or PHS) is a collection of agencies of the Department of Health and Human Services concerned with public health, containing nine out of the department's twelve operating divisions. The Assistant ...
, in 1925, based on field data from the
Ohio River
The Ohio River is a long river in the United States. It is located at the boundary of the Midwestern and Southern United States, flowing southwesterly from western Pennsylvania to its mouth on the Mississippi River at the southern tip of Illino ...
. The equation is also known as the DO sag equation.
Streeter–Phelps equation
The Streeter–Phelps equation determines the relation between the
dissolved oxygen
Oxygen saturation (symbol SO2) is a relative measure of the concentration of oxygen that is dissolved or carried in a given medium as a proportion of the maximal concentration that can be dissolved in that medium at the given temperature. It ca ...
concentration and the
biological oxygen demand
Biochemical oxygen demand (BOD) is the amount of dissolved oxygen (DO) needed (i.e. demanded) by aerobic biological organisms to break down organic material present in a given water sample at a certain temperature over a specific time period. T ...
over time and is a solution to the linear first order differential equation
[Streeter H. W., Phelps E. B., 1925, A Study of the pollution and natural purification of the Ohio river. III. Factors concerned in the phenomena of oxidation and reaeration, Public Health Bulletin no. 146, Reprinted by U.S. Department of Health, Education and Welfare, Public Health Service, 1958, ISBN B001BP4GZI, http://dspace.udel.edu:8080/dspace/bitstream/handle/19716/1590/C%26EE148.pdf?sequence=2]
:
This differential equation states that the total change in oxygen deficit (D) is equal to the difference between the two rates of
deoxygenation
Deoxygenation is a chemical reaction involving the removal of oxygen atoms from a molecule. The term also refers to the removal of molecular oxygen (O2) from gases and solvents, a step in air-free technique and gas purifiers. As applied to orga ...
and reaeration at any time.
The Streeter–Phelps equation, assuming a plug-flow stream at steady state is then
:
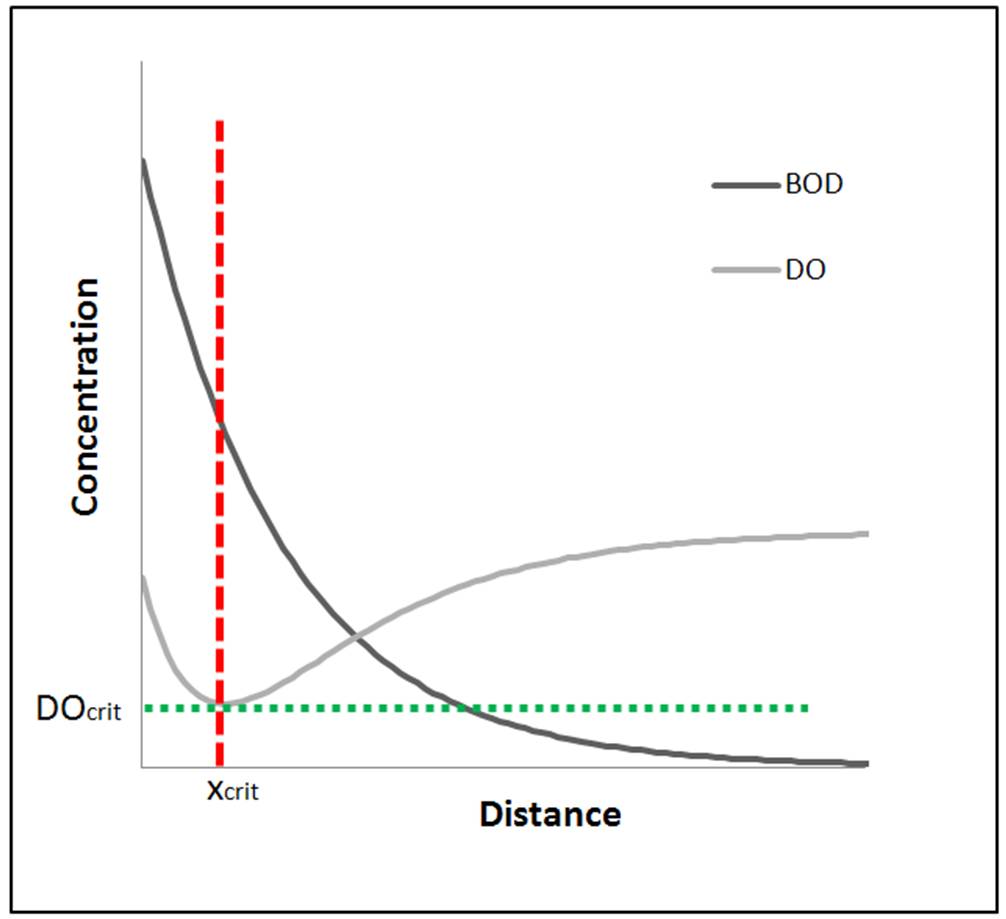
where
*
is the saturation deficit, which can be derived from the dissolved oxygen concentration at saturation minus the actual dissolved oxygen concentration (
).
has the dimensions
.
*
is the
deoxygenation
Deoxygenation is a chemical reaction involving the removal of oxygen atoms from a molecule. The term also refers to the removal of molecular oxygen (O2) from gases and solvents, a step in air-free technique and gas purifiers. As applied to orga ...
rate, usually in
.
*
is the reaeration rate, usually in
.
*
is the initial oxygen demand of organic matter in the water, also called the ultimate BOD (BOD at time t=infinity). The unit of
is
.
*
is the oxygen demand remaining at time t,
.
*
is the initial oxygen deficit
 The Streeter–Phelps equation is used in the study of
The Streeter–Phelps equation is used in the study of  where
* is the saturation deficit, which can be derived from the dissolved oxygen concentration at saturation minus the actual dissolved oxygen concentration (). has the dimensions .
* is the
where
* is the saturation deficit, which can be derived from the dissolved oxygen concentration at saturation minus the actual dissolved oxygen concentration (). has the dimensions .
* is the
 where
* is the saturation deficit, which can be derived from the dissolved oxygen concentration at saturation minus the actual dissolved oxygen concentration (). has the dimensions .
* is the
where
* is the saturation deficit, which can be derived from the dissolved oxygen concentration at saturation minus the actual dissolved oxygen concentration (). has the dimensions .
* is the