square planar molecular geometry on:
[Wikipedia]
[Google]
[Amazon]
 The square planar molecular geometry in chemistry describes the stereochemistry (spatial arrangement of atoms) that is adopted by certain
The square planar molecular geometry in chemistry describes the stereochemistry (spatial arrangement of atoms) that is adopted by certain
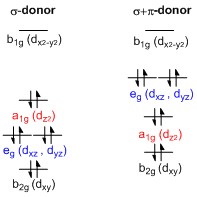 A general d-orbital splitting diagram for square planar (D4h) transition metal complexes can be derived from the general octahedral (Oh) splitting diagram, in which the d''z''2 and the d''x''2−''y''2 orbitals are degenerate and higher in energy than the degenerate set of dxy, dxz and dyz orbitals. When the two axial ligands are removed to generate a square planar geometry, the d''z''2 orbital is driven lower in energy as electron-electron repulsion with ligands on the z-axis is no longer present. However, for purely σ-donating ligands the d''z''2 orbital is still higher in energy than the dxy, dxz and dyz orbitals because of the
A general d-orbital splitting diagram for square planar (D4h) transition metal complexes can be derived from the general octahedral (Oh) splitting diagram, in which the d''z''2 and the d''x''2−''y''2 orbitals are degenerate and higher in energy than the degenerate set of dxy, dxz and dyz orbitals. When the two axial ligands are removed to generate a square planar geometry, the d''z''2 orbital is driven lower in energy as electron-electron repulsion with ligands on the z-axis is no longer present. However, for purely σ-donating ligands the d''z''2 orbital is still higher in energy than the dxy, dxz and dyz orbitals because of the
3D Chem
– Chemistry, Structures, and 3D Molecules
IUMSC
– Indiana University Molecular Structure Center
– Coordination numbers and complex ions {{DEFAULTSORT:Square Planar Molecular Geometry Stereochemistry Molecular geometry
 The square planar molecular geometry in chemistry describes the stereochemistry (spatial arrangement of atoms) that is adopted by certain
The square planar molecular geometry in chemistry describes the stereochemistry (spatial arrangement of atoms) that is adopted by certain chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one ele ...
s. As the name suggests, molecules of this geometry have their atoms positioned at the corners.
Examples
Numerous compounds adopt this geometry, examples being especially numerous for transition metal complexes. Thenoble gas
The noble gases (historically also the inert gases; sometimes referred to as aerogens) make up a class of chemical elements with similar properties; under standard conditions, they are all odorless, colorless, monatomic gases with very low che ...
compound XeF4 adopts this structure as predicted by VSEPR theory
Valence shell electron pair repulsion (VSEPR) theory ( , ), is a model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. It is also named the Gillespie-Nyholm t ...
. The geometry is prevalent for transition metal complexes with d8 configuration, which includes Rh(I), Ir(I), Pd(II), Pt(II), and Au(III). Notable examples include the anticancer drugs cisplatin
Cisplatin is a chemotherapy medication used to treat a number of cancers. These include testicular cancer, ovarian cancer, cervical cancer, breast cancer, bladder cancer, head and neck cancer, esophageal cancer, lung cancer, mesothelio ...
tCl2(NH3)2and carboplatin
Carboplatin, sold under the trade name Paraplatin among others, is a chemotherapy medication used to treat a number of forms of cancer. This includes ovarian cancer, lung cancer, head and neck cancer, brain cancer, and neuroblastoma. It is used ...
. Many homogeneous catalysts are square planar in their resting state, such as Wilkinson's catalyst and Crabtree's catalyst. Other examples include Vaska's complex and Zeise's salt. Certain ligands (such as porphyrin
Porphyrins ( ) are a group of heterocyclic macrocycle organic compounds, composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges (=CH−). The parent of porphyrin is porphine, a rare chemical ...
s) stabilize this geometry.
Splitting of d-orbitals
 A general d-orbital splitting diagram for square planar (D4h) transition metal complexes can be derived from the general octahedral (Oh) splitting diagram, in which the d''z''2 and the d''x''2−''y''2 orbitals are degenerate and higher in energy than the degenerate set of dxy, dxz and dyz orbitals. When the two axial ligands are removed to generate a square planar geometry, the d''z''2 orbital is driven lower in energy as electron-electron repulsion with ligands on the z-axis is no longer present. However, for purely σ-donating ligands the d''z''2 orbital is still higher in energy than the dxy, dxz and dyz orbitals because of the
A general d-orbital splitting diagram for square planar (D4h) transition metal complexes can be derived from the general octahedral (Oh) splitting diagram, in which the d''z''2 and the d''x''2−''y''2 orbitals are degenerate and higher in energy than the degenerate set of dxy, dxz and dyz orbitals. When the two axial ligands are removed to generate a square planar geometry, the d''z''2 orbital is driven lower in energy as electron-electron repulsion with ligands on the z-axis is no longer present. However, for purely σ-donating ligands the d''z''2 orbital is still higher in energy than the dxy, dxz and dyz orbitals because of the torus
In geometry, a torus (plural tori, colloquially donut or doughnut) is a surface of revolution generated by revolving a circle in three-dimensional space about an axis that is coplanar with the circle.
If the axis of revolution does not ...
shaped lobe of the d''z''2 orbital. It bears electron density on the x- and y-axes and therefore interacts with the filled ligand orbitals. The dxy, dxz and dyz orbitals are generally presented as degenerate but they have to split into two different energy levels with respect to the irreducible representations of the point group
In geometry, a point group is a mathematical group of symmetry operations ( isometries in a Euclidean space) that have a fixed point in common. The coordinate origin of the Euclidean space is conventionally taken to be a fixed point, and every ...
D4h. Their relative ordering depends on the nature of the particular complex. Furthermore, the splitting of d-orbitals is perturbed by π-donating ligands in contrast to octahedral complexes. In the square planar case strongly π-donating ligands can cause the dxz and dyz orbitals to be higher in energy than the d''z''2 orbital, whereas in the octahedral case π-donating ligands only affect the magnitude of the d-orbital splitting and the relative ordering of the orbitals is conserved.
See also
* AXE method *Molecular geometry
Molecular geometry is the three-dimensional space, three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometric ...
References
External links
3D Chem
– Chemistry, Structures, and 3D Molecules
IUMSC
– Indiana University Molecular Structure Center
– Coordination numbers and complex ions {{DEFAULTSORT:Square Planar Molecular Geometry Stereochemistry Molecular geometry