sodium polystyrene sulfonate on:
[Wikipedia]
[Google]
[Amazon]
Polystyrene sulfonates are a group of medications used to treat high blood potassium. Effects generally take hours to days. They are also used to remove
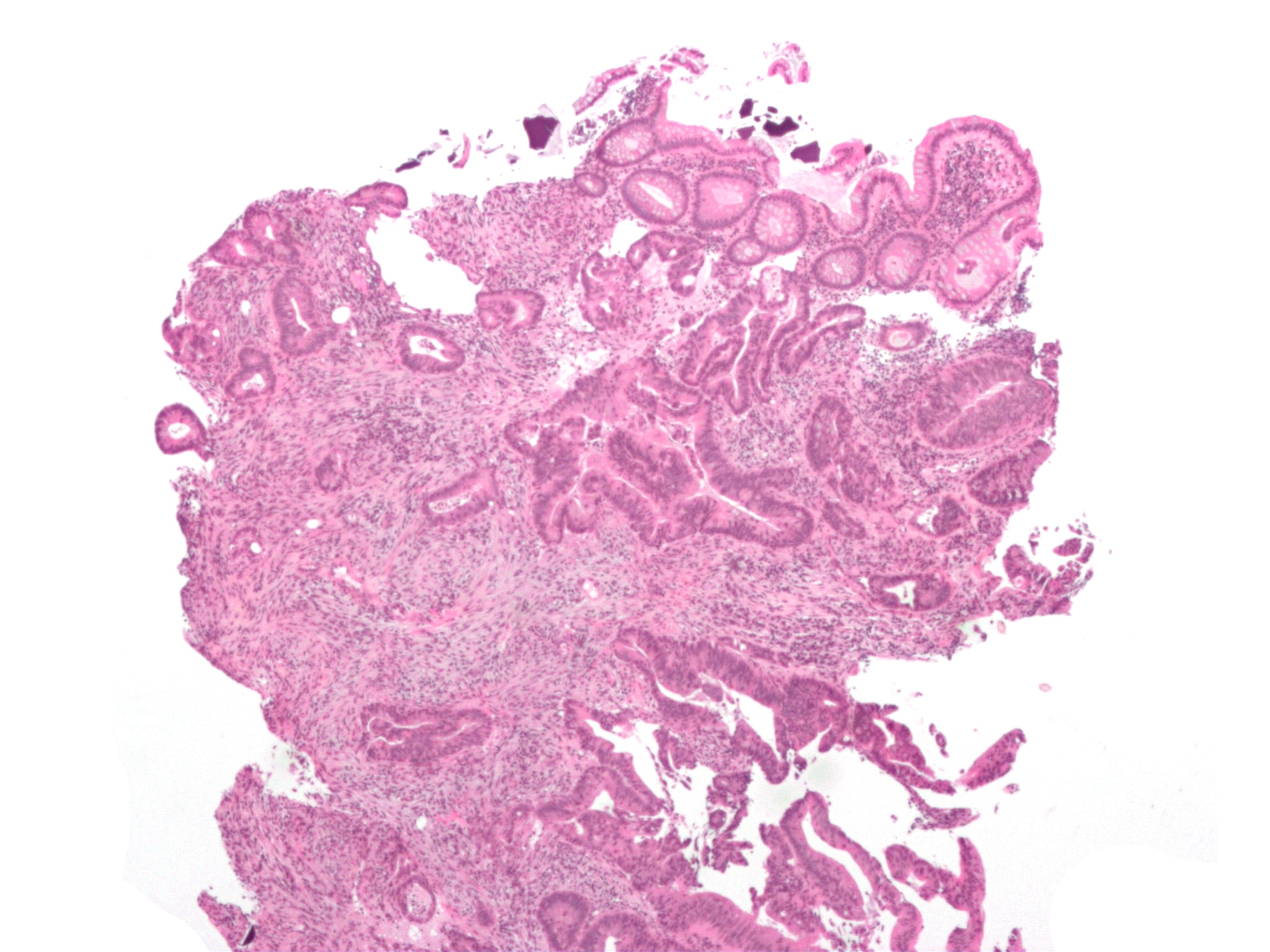 Polystyrene sulfonate is usually supplied in either the sodium or calcium form. It is used as a potassium binder in acute and
Polystyrene sulfonate is usually supplied in either the sodium or calcium form. It is used as a potassium binder in acute and

potassium
Potassium is a chemical element; it has Symbol (chemistry), symbol K (from Neo-Latin ) and atomic number19. It is a silvery white metal that is soft enough to easily cut with a knife. Potassium metal reacts rapidly with atmospheric oxygen to ...
, calcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to it ...
, and sodium
Sodium is a chemical element; it has Symbol (chemistry), symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 element, group 1 of the peri ...
from solutions in technical applications.
Common side effects include loss of appetite, gastrointestinal upset, constipation, and low blood calcium. These polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
s are derived from polystyrene
Polystyrene (PS) is a synthetic polymer made from monomers of the aromatic hydrocarbon styrene. Polystyrene can be solid or foamed. General-purpose polystyrene is clear, hard, and brittle. It is an inexpensive resin per unit weight. It i ...
by the addition of sulfonate
In organosulfur chemistry, a sulfonate is a salt, anion or ester of a sulfonic acid. Its formula is , containing the functional group , where R is typically an organyl group, amino group or a halogen atom. Sulfonates are the conjugate bases of ...
functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
s.
Sodium polystyrene sulfonate was approved for medical use in the United States in 1958.
A polystyrene sulfonate was developed in the 2000s to treat '' Clostridioides difficile'' associated diarrhea under the name Tolevamer, but it was never marketed.
Medical uses
 Polystyrene sulfonate is usually supplied in either the sodium or calcium form. It is used as a potassium binder in acute and
Polystyrene sulfonate is usually supplied in either the sodium or calcium form. It is used as a potassium binder in acute and chronic kidney disease
Chronic kidney disease (CKD) is a type of long-term kidney disease, defined by the sustained presence of abnormal kidney function and/or abnormal kidney structure. To meet criteria for CKD, the abnormalities must be present for at least three mo ...
for people with hyperkalemia
Hyperkalemia is an elevated level of potassium (K+) in the blood. Normal potassium levels are between 3.5 and 5.0 mmol/L (3.5 and 5.0 mEq/L) with levels above 5.5mmol/L defined as hyperkalemia. Typically hyperkalemia does not cause symptoms. Oc ...
(an abnormally high blood serum potassium level). However, it is unclear if it is beneficial and there is concern about possible side effects when it is combined with sorbitol
Sorbitol (), less commonly known as glucitol (), is a sugar alcohol with a sweet taste which the human body metabolizes slowly. It can be obtained by reduction of glucose, which changes the converted aldehyde group (−CHO) to a primary alco ...
.
Polystyrene sulfonates are given by mouth with a meal or rectally by retention enema
An enema, also known as a clyster, is the rectal administration of a fluid by injection into the Large intestine, lower bowel via the anus.Cullingworth, ''A Manual of Nursing, Medical and Surgical'':155 The word ''enema'' can also refer to the ...
.
Side effects
Intestinal disturbances are common, including loss of appetite,nausea
Nausea is a diffuse sensation of unease and discomfort, sometimes perceived as an urge to vomit. It can be a debilitating symptom if prolonged and has been described as placing discomfort on the chest, abdomen, or back of the throat.
Over 30 d ...
, vomiting
Vomiting (also known as emesis, puking and throwing up) is the forceful expulsion of the contents of one's stomach through the mouth and sometimes the nose.
Vomiting can be the result of ailments like food poisoning, gastroenteritis, pre ...
, and constipation
Constipation is a bowel dysfunction that makes bowel movements infrequent or hard to pass. The Human feces, stool is often hard and dry. Other symptoms may include abdominal pain, bloating, and feeling as if one has not completely passed the ...
. In rare cases, it has been associated with colonic necrosis. Changes in electrolyte blood levels such as hypomagnesemia, hypocalcemia, and hypokalemia may occur. Polystyrene sulfonates should not be used in people with obstructive bowel disease and in newborns with reduced gut motility. for Kayexalate.
Intestinal injury
A total of 58 cases of intestinal injury includingnecrosis
Necrosis () is a form of cell injury which results in the premature death of cells in living tissue by autolysis. The term "necrosis" came about in the mid-19th century and is commonly attributed to German pathologist Rudolf Virchow, who i ...
of the colon have been reported with polystyrene sulfonate as of 2013. Well more cases have been reported when used in combination with sorbitol and other cases have occurred when used alone.
Interactions
Polystyrene sulfonates can bind to various drugs within the digestive tract and thus lower their absorption and effectiveness. Common examples includelithium
Lithium (from , , ) is a chemical element; it has chemical symbol, symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard temperature and pressure, standard conditions, it is the least dense metal and the ...
, thyroxine
Thyroxine, also known as T4, is a hormone produced by the thyroid gland. It is the primary form of thyroid hormone found in the blood and acts as a prohormone of the more active thyroid hormone, triiodothyronine (T3). Thyroxine and its acti ...
, and digitalis
''Digitalis'' ( or ) is a genus of about 20 species of herbaceous perennial plants, shrubs, and Biennial plant, biennials, commonly called foxgloves.
''Digitalis'' is native to Europe, Western Asia, and northwestern Africa. The flowers are ...
. In September 2017, the FDA recommended separating the dosing of polystyrene sulfonate from any other oral medications by at least three hours to avoid any potential interactions.
Mechanism of action
Hyperkalemia
Polystyrene sulfonates release sodium or calcium ions in the stomach in exchange for hydrogen ions. When the resin reaches the large intestine the hydrogen ions are exchanged for free potassium ions, and the resin is then eliminated in the feces. The net effect is lowering the amount of potassium available for absorption into the blood and increasing the amount that is excreted via the feces. The effect is a reduction of potassium levels in the body, at a capacity of 1 mEq of potassium exchanged per 1 g of resin.Production and chemical structure
Polystyrene sulfonic acid, the acid whose salts are the polystyrene sulfonates, has the idealized formula (CH2CHC6H4SO3H)''n''. The material is prepared by sulfonation ofpolystyrene
Polystyrene (PS) is a synthetic polymer made from monomers of the aromatic hydrocarbon styrene. Polystyrene can be solid or foamed. General-purpose polystyrene is clear, hard, and brittle. It is an inexpensive resin per unit weight. It i ...
:
:(CH2CHC6H5)''n'' + ''n'' SO3 → (CH2CHC6H4SO3H)''n''
Several methods exist for this conversion, which can lead to varying degree of sulfonation. Usually the polystyrene is crosslinked
In chemistry and biology, a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural ...
, which keeps the polymer from dissolving. Since the sulfonic acid
In organic chemistry, sulfonic acid (or sulphonic acid) refers to a member of the class of organosulfur compounds with the general formula , where R is an organic alkyl or aryl group and the group a sulfonyl hydroxide. As a substituent, it is kn ...
group (SO3H) is strongly acidic, this polymer neutralizes bases. In this way, various salts of the polymer can be prepared, leading to sodium, calcium, and other salts:
:(CH2CHC6H4SO3H)''n'' + ''n'' NaOH → (CH2CHC6H4SO3Na)''n'' + ''n'' H2O
These ion-containing polymers are called ionomers.
Alternative sulfonation methods
Double substitutions of the phenyl rings are known to occur, even with conversions well below 100%. Crosslinking reactions are also found, where condensation of two sulfonic acid groups yields a sulfonyl crosslink. On the other hand, the use of milder conditions such as acetyl sulfate leads to incomplete sulfonation. Recently, the atom transfer radical polymerization (ATRP) of protected styrene sulfonates has been reported, leading to well defined linear polymers, as well as more complicated molecular architectures.Chemical uses
Polystyrene sulfonates are useful because of theirion exchange
Ion exchange is a reversible interchange of one species of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid. Ion exchange is used in softening or demineralizing of water, purification of ch ...
properties. Linear ionic polymers are generally water-soluble
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solub ...
, whereas cross-link
In chemistry and biology, a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural ...
ed materials (called resin
A resin is a solid or highly viscous liquid that can be converted into a polymer. Resins may be biological or synthetic in origin, but are typically harvested from plants. Resins are mixtures of organic compounds, predominantly terpenes. Commo ...
s) do not dissolve in water. These polymers are classified as polysalts and ionomers.
Water softening
Water softening is achieved by percolating hard water through a bed of the sodium form of cross-linked polystyrene sulfonate. The hard ions such ascalcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to it ...
(Ca2+) and magnesium
Magnesium is a chemical element; it has Symbol (chemistry), symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 ...
(Mg2+) adhere to the sulfonate groups, displacing sodium ions. The resulting solution of sodium ions is softened.

Other uses
Sodium polystyrene sulfonate is used as a superplastifier in cement, as a dye improving agent for cotton, and as proton exchange membranes in fuel cell applications. In its acid form, the resin is used as a solid acid catalyst inorganic synthesis
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the gen ...
, mostly commonly under the tradename Amberlyst.
References
{{Drugs for treatment of hyperkalemia and hyperphosphatemia Benzenesulfonates Nephrology procedures Organic polymers Plastics Polyelectrolytes Chelating agents used as drugs Acid catalysts Vinyl polymers Sanofi