Silicate Brick on:
[Wikipedia]
[Google]
[Amazon]
 In
In

 With two shared oxides bound to each silicon, cyclic or polymeric structures can result. The cyclic
With two shared oxides bound to each silicon, cyclic or polymeric structures can result. The cyclic 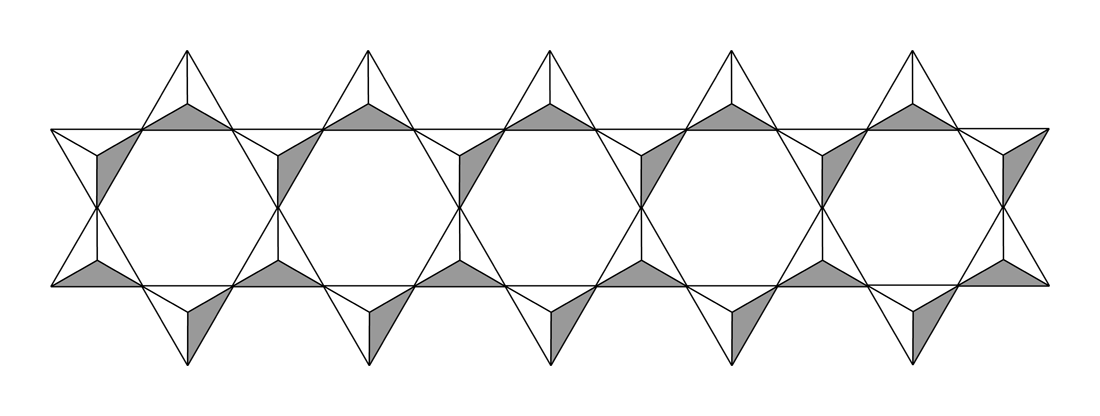 Double-chain silicates, the other category of inosilicates, occur when tetrahedra form a double chain (not always but mostly) by sharing two or three oxygen atoms each. Common minerals for this group are amphiboles.
Double-chain silicates, the other category of inosilicates, occur when tetrahedra form a double chain (not always but mostly) by sharing two or three oxygen atoms each. Common minerals for this group are amphiboles.
 In this group, known as phyllosilicates, tetrahedra all share three oxygen atoms each and in turn link to form two-dimensional sheets. This structure does lead to minerals in this group having one strong cleavage plane. Micas fall into this group. Both muscovite and
In this group, known as phyllosilicates, tetrahedra all share three oxygen atoms each and in turn link to form two-dimensional sheets. This structure does lead to minerals in this group having one strong cleavage plane. Micas fall into this group. Both muscovite and
 In
In chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ...
, a silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate
In chemistry, orthosilicate is the anion , or any of its salts and esters. It is one of the silicate anions. It is occasionally called the silicon tetroxide anion or group.C. A. Kumins, and A. E. Gessler (1953), "Short-Cycle Syntheses of Ultrama ...
(), metasilicate 320 px, Idealized structure of sodium metasilicate.
Metasilicates are silicates containing ions of empirical formula . Common stoichiometries include MSiO3 and MIISiO3. Metasilicates can be cyclic, usually the hexamer or chains .
Common comp ...
(), and pyrosilicate
A pyrosilicate is a type of chemical compound; either an ionic compound that contains the pyrosilicate anion , or an organic compound with the hexavalent ≡-O-≡ group. The anion is also called disilicateViktor Renman (2017): "Structural and E ...
(, ). The name is also used for any salt of such anions, such as sodium metasilicate; or any ester containing the corresponding chemical group, such as tetramethyl orthosilicate. The name "silicate" is sometimes extended to any anions containing silicon, even if they do not fit the general formula or contain other atoms besides oxygen; such as hexafluorosilicate .Most commonly, silicates are encountered as silicate minerals.
For diverse manufacturing, technological, and artistic needs, silicates are versatile materials, both natural (such as granite, gravel
Gravel is a loose aggregation of rock fragments. Gravel occurs naturally throughout the world as a result of sedimentary and erosive geologic processes; it is also produced in large quantities commercially as crushed stone.
Gravel is classifi ...
, and garnet) and artificial (such as Portland cement, ceramics, glass, and waterglass).
Structural principles
In all silicates, silicon atom occupies the center of an idealized tetrahedron whose corners are four oxygen atoms, connected to it by singlecovalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
s according to the octet rule. The oxygen atoms, which bears some negative charge, link to other cations (Mn+). This Si-O-M-O-Si linkage is strong and rigid, which properties are manifested in the rock-like silicates. The silicates can be classified according to the length and crosslinking of the silicate anions.Isolated silicates
Isolatedorthosilicate
In chemistry, orthosilicate is the anion , or any of its salts and esters. It is one of the silicate anions. It is occasionally called the silicon tetroxide anion or group.C. A. Kumins, and A. E. Gessler (1953), "Short-Cycle Syntheses of Ultrama ...
anions have the formula . A common mineral in this group is olivine ().
Ttwo or more silicon atoms can share oxygen atoms in various ways, to form more complex anions, such as pyrosilicate
A pyrosilicate is a type of chemical compound; either an ionic compound that contains the pyrosilicate anion , or an organic compound with the hexavalent ≡-O-≡ group. The anion is also called disilicateViktor Renman (2017): "Structural and E ...
.
Chains

 With two shared oxides bound to each silicon, cyclic or polymeric structures can result. The cyclic
With two shared oxides bound to each silicon, cyclic or polymeric structures can result. The cyclic metasilicate 320 px, Idealized structure of sodium metasilicate.
Metasilicates are silicates containing ions of empirical formula . Common stoichiometries include MSiO3 and MIISiO3. Metasilicates can be cyclic, usually the hexamer or chains .
Common comp ...
ring is a hexamer of SiO32-. Polymeric silicate anions of can exist also as long chains.
In single-chain silicates, which are a type of inosilicate, tetrahedra link to form a chain by sharing two oxygen atoms each. A common mineral in this group is pyroxene
The pyroxenes (commonly abbreviated to ''Px'') are a group of important rock-forming inosilicate minerals found in many igneous and metamorphic rocks. Pyroxenes have the general formula , where X represents calcium (Ca), sodium (Na), iron (Fe II) ...
.
 Double-chain silicates, the other category of inosilicates, occur when tetrahedra form a double chain (not always but mostly) by sharing two or three oxygen atoms each. Common minerals for this group are amphiboles.
Double-chain silicates, the other category of inosilicates, occur when tetrahedra form a double chain (not always but mostly) by sharing two or three oxygen atoms each. Common minerals for this group are amphiboles.
Sheets
 In this group, known as phyllosilicates, tetrahedra all share three oxygen atoms each and in turn link to form two-dimensional sheets. This structure does lead to minerals in this group having one strong cleavage plane. Micas fall into this group. Both muscovite and
In this group, known as phyllosilicates, tetrahedra all share three oxygen atoms each and in turn link to form two-dimensional sheets. This structure does lead to minerals in this group having one strong cleavage plane. Micas fall into this group. Both muscovite and biotite
Biotite is a common group of phyllosilicate minerals within the mica group, with the approximate chemical formula . It is primarily a solid-solution series between the iron-endmember annite, and the magnesium-endmember phlogopite; more alumino ...
have very weak layers that can be peeled off in sheets.
Framework
In a framework silicate, known as a tectosilicate, each tetrahedron shares all 4 oxygen atoms with its neighbours, forming a 3D structure. Quartz and feldspars are in this group.Silicates with non-tetrahedral silicon
Although the tetrahedron is a common coordination geometry for silicon(IV) compounds, silicon may also occur with higher coordination numbers. For example, in the anion hexafluorosilicate , the silicon atom is surrounded by sixfluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reacti ...
atoms in an octahedral arrangement. This structure is also seen in the hexahydroxysilicate anion that occurs in thaumasite, a mineral found rarely in nature but sometimes observed among other calcium silicate hydrates artificially formed in cement and concrete submitted to a severe sulfate attack
Cement hydration and strength development mainly depend on two silicate phases: tricalcium silicate (C3S) (alite), and dicalcium silicate (C2S) (belite). Upon hydration, the main reaction products are calcium silicate hydrates (C-S-H) and calciu ...
.
At very high pressure, such as exists in the majority of the earth's crust, even SiO2 adopts the six-coordinated octahedral geometry in the mineral stishovite
Stishovite is an extremely hard, dense tetragonal form (Polymorphism (materials science), polymorph) of silicon dioxide. It is very rare on the Earth's surface; however, it may be a predominant form of silicon dioxide in the Earth, especially in ...
, a dense polymorph of silica found in the lower mantle
The lower mantle, historically also known as the mesosphere, represents approximately 56% of Earth's total volume, and is the region from 660 to 2900 km below Earth's surface; between the transition zone and the outer core. The preliminar ...
of the Earth and also formed by shock during meteorite
A meteorite is a solid piece of debris from an object, such as a comet, asteroid, or meteoroid, that originates in outer space and survives its passage through the atmosphere to reach the surface of a planet or Natural satellite, moon. When the ...
impacts.
Chemical properties
Silicates withalkali
In chemistry, an alkali (; from ar, القلوي, al-qaly, lit=ashes of the saltwort) is a basic, ionic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a ...
cations and small or chain-like anions, such as sodium ortho- and metasilicate 320 px, Idealized structure of sodium metasilicate.
Metasilicates are silicates containing ions of empirical formula . Common stoichiometries include MSiO3 and MIISiO3. Metasilicates can be cyclic, usually the hexamer or chains .
Common comp ...
, are fairly soluble in water. They form several solid hydrate
In chemistry, a hydrate is a substance that contains water or its constituent elements. The chemical state of the water varies widely between different classes of hydrates, some of which were so labeled before their chemical structure was understo ...
s when crystallized from solution. Soluble sodium silicate
Sodium silicate is a generic name for chemical compounds with the formula or ·, such as sodium metasilicate , sodium orthosilicate , and sodium pyrosilicate . The anions are often polymeric. These compounds are generally colorless transparent ...
s and mixtures thereof, known as waterglass are in fact important industrial and household chemicals. Silicates of non-alkali cations, or with sheet and tridimensional polymeric anions, generally have negligible solubility in water at normal conditions.
Reactions
Silicates are generally inert chemically. Hence they are common minerals. Their resiliency also recommends their use as building materials. When treated with calcium oxides and water, silicate minerals form Portland cement. Equilibria involving hydrolysis of silicate minerals are difficult to study. The chief challenge is the very low solubility of SiO44- and its various protonated forms. Such equilibria are relevant to the processes occurring on geological time scales.G. B. Alexander (1953): "The Reaction of Low Molecular Weight Silicic Acids with Molybdic Acid". ''Journal of the American Chemical Society, volume 75, issue 22, pages 5655–5657. Some plants excrete ligands that dissolve silicates, a step in biomineralization.Detection
Silicate anions in solution react withmolybdate
In chemistry a molybdate is a compound containing an oxoanion with molybdenum in its highest oxidation state of 6. Molybdenum can form a very large range of such oxoanions which can be discrete structures or polymeric extended structures, althoug ...
anions yielding yellow silicomolybdate complexes. In a typical preparation, monomeric orthosilicate was found to react completely in 75 seconds; dimer
Dimer may refer to:
* Dimer (chemistry), a chemical structure formed from two similar sub-units
** Protein dimer, a protein quaternary structure
** d-dimer
* Dimer model, an item in statistical mechanics, based on ''domino tiling''
* Julius Dimer ( ...
ic pyrosilicate in 10 minutes; and higher oligomer
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relativ ...
s in considerably longer time. In particular, the reaction is not observed with suspensions of colloidal silica.
Zeolite formation
The nature of soluble silicates is relevant to understanding biomineralization and the synthesis of aluminosilicates, such as the industrially important catalysts called zeolites.See also
* * Alkali-silica reaction * Carbon cycle * Carbonate-silicate cycle * Ocean acidificationReferences
{{DEFAULTSORT:Silicon-oxygen tetrahedron Silicates