Salicylaldoxime on:
[Wikipedia]
[Google]
[Amazon]
Salicylaldoxime is an
Chemical data at NIST Chemistry WebBook
organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon- hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. Th ...
described by the formula C6H4CH=NOH-2-OH. It is the oxime
In organic chemistry, an oxime is a organic compound belonging to the imines, with the general formula , where R is an organic side-chain and R’ may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substitu ...
of salicylaldehyde
Salicylic aldehyde (2-hydroxybenzaldehyde) is the organic compound with the formula (C7 H6 O2) C6H4CHO-2-OH. Along with 3-hydroxybenzaldehyde and 4-hydroxybenzaldehyde, it is one of the three isomers of hydroxybenzaldehyde. This colorless oi ...
. This crystalline, colorless solid is a chelator
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are ...
and sometimes used in the analysis of samples containing transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that c ...
ions, with which it often forms brightly coloured coordination complex
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many ...
es.
Reactions
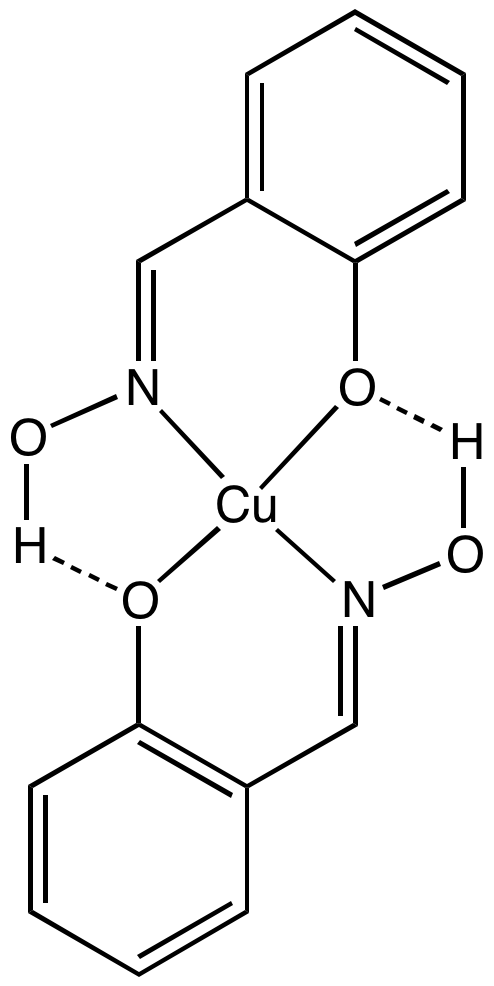
Salicylaldoxime is the conjugate acid of a bidentate ligand: :2 C6H4CH=NOH-2-OH + Cu2+ → Cu(C6H4CH=NOH-2-O)2 + 2 H+ In highly acidic media, the ligand decomplexes and the metal aqua complex is liberated. In this way the ligand is used as a recyclable extractant. It typically forms charge-neutral complexes with divalent metal ions.Analytical chemistry
In the era when metals were analysed byspectrophotometry
Spectrophotometry is a branch of electromagnetic spectroscopy concerned with the quantitative measurement of the reflection or transmission properties of a material as a function of wavelength. Spectrophotometry uses photometers, known as sp ...
, many chelating ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's ele ...
s were developed that selectively formed brightly coloured complexes with particular metal ions. This methodology has been eclipsed with the introduction of inductively coupled plasma methodology. Salicylaldoxime can be used to selectively precipitate
In an aqueous solution, precipitation is the process of transforming a dissolved substance into an insoluble solid from a super-saturated solution. The solid formed is called the precipitate. In case of an inorganic chemical reaction leading ...
metal ions for gravimetric determination. It forms a greenish-yellow precipitate with copper at a pH of 2.6 in the presence of acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main componen ...
. Under these conditions, this is the only metal that precipitates; at pH 3.3, nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow ...
also precipitates. Iron (III) will interfere.
It has been used as an ionophore in ion selective electrode
An ion-selective electrode (ISE), also known as a specific ion electrode (SIE), is a transducer (or sensor) that converts the activity of a specific ion dissolved in a solution into an electrical potential. The voltage is theoretically dependent o ...
s, with good response to Pb2+ and Ni2+.

Extraction of metals
Saloximes are used in the extraction and separation of metals from their ores. In one application of hydrometallurgy, Cu2+ is extracted into organic solvents as its saloxime complex.Peter A. Tasker, Christine C. Tong, Arjan N. Westra "Co-extraction of cations and anions in base metal recovery" Coordination Chemistry Reviews 2007, vol. 251, pp. 1868–1877. {{doi, 10.1016/j.ccr.2007.03.014External links
Chemical data at NIST Chemistry WebBook
References