SRN1 Mechanism on:
[Wikipedia]
[Google]
[Amazon]
Radical-nucleophilic aromatic substitution or SRN1 in  The substituent X is a
The substituent X is a
 In this
In this  The
The 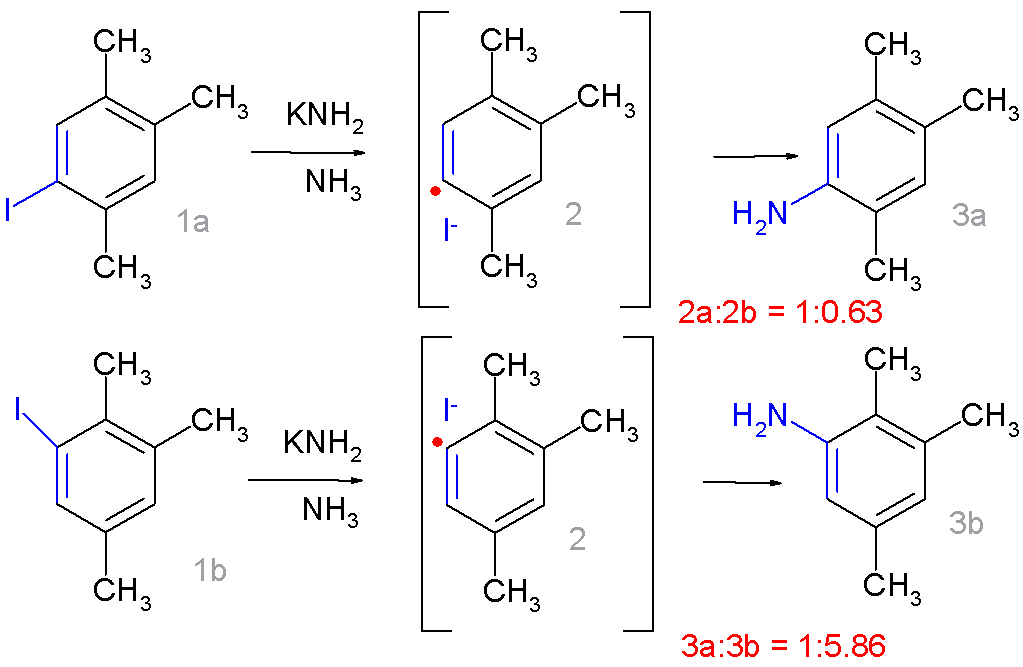 It now resembles ''ipso''-substitution with 1a forming preferentially 3a and 1b forming 3b. Radical scavengers suppress ''ipso''-substitution in favor of ''cine''-substitution and the addition of potassium metal as an electron donor and
It now resembles ''ipso''-substitution with 1a forming preferentially 3a and 1b forming 3b. Radical scavengers suppress ''ipso''-substitution in favor of ''cine''-substitution and the addition of potassium metal as an electron donor and
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clay ...
is a type of substitution reaction
A substitution reaction (also known as single displacement reaction or single substitution reaction) is a chemical reaction during which one functional group in a chemical compound is replaced by another functional group. Substitution reactions ar ...
in which a certain substituent
A substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule. (In organic chemistry and biochemistry, the terms ''substituent'' and '' functional group'', as well as '' ...
on an aromatic compound
Aromatic compounds, also known as "mono- and polycyclic aromatic hydrocarbons", are organic compounds containing one or more aromatic rings. The parent member of aromatic compounds is benzene. The word "aromatic" originates from the past grouping ...
is replaced by a nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they a ...
through an intermediary free radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Ailments of unknown cause
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabo ...
species:
 The substituent X is a
The substituent X is a halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a f ...
and nucleophiles can be sodium amide
Sodium amide, commonly called sodamide (systematic name sodium azanide), is the inorganic compound with the formula . It is a salt composed of the sodium cation and the azanide anion. This solid, which is dangerously reactive toward water, is whit ...
, an alkoxide or a carbon nucleophile such as an enolate
In organic chemistry, enolates are organic anions derived from the deprotonation of carbonyl () compounds. Rarely isolated, they are widely used as reagents in the synthesis of organic compounds.
Bonding and structure
Enolate anions are electr ...
. In contrast to regular nucleophilic aromatic substitution
A nucleophilic aromatic substitution is a substitution reaction in organic chemistry in which the nucleophile displaces a good leaving group, such as a halide, on an aromatic ring. Aromatic rings are usually nucleophilic, but some aromatic compoun ...
, deactivating group In electrophilic aromatic substitution reactions, existing substituent groups on the aromatic ring influence the overall reaction rate or have a directing effect on positional isomer of the products that are formed. An electron donating group (ED ...
s on the arene are not required.
This reaction type was discovered in 1970 by Bunnett and Kim and the abbreviation SRN1 stands for substitution radical-nucleophilic unimolecular as it shares properties with an aliphatic SN1 reaction. An example of this reaction type is the Sandmeyer reaction.
Reaction mechanism
 In this
In this radical substitution
In organic chemistry, a radical-substitution reaction is a substitution reaction involving free radicals as a reactive intermediate.March Jerry; (1985). Advanced organic chemistry reactions, mechanisms and structure (3rd ed.). New York: John Wiley ...
the aryl halide 1 accepts an electron from a radical initiator
In chemistry, radical initiators are substances that can produce radical species under mild conditions and promote radical reactions. These substances generally possess weak bonds—bonds that have small bond dissociation energies. Radical i ...
forming a radical anion
In organic chemistry, a radical anion is a free radical species that carries a negative charge. Radical anions are encountered in organic chemistry as reduced derivatives of polycyclic aromatic compounds, e.g. sodium naphthenide. An example of a ...
2. This intermediate collapses into an aryl radical 3 and a halide anion. The aryl radical reacts with the nucleophile 4 to a new radical anion 5 which goes on to form the substituted product by transferring its electron to new aryl halide in the chain propagation. Alternatively the phenyl radical can abstract any loose proton from 7 forming the arene 8 in a chain termination
Chain termination is any chemical reaction that ceases the formation of reactive intermediates in a chain propagation step in the course of a polymerization, effectively bringing it to a halt.
Mechanisms of termination
In polymer chemist ...
reaction.
The involvement of a radical intermediate in a new type of nucleophilic aromatic substitution
A nucleophilic aromatic substitution is a substitution reaction in organic chemistry in which the nucleophile displaces a good leaving group, such as a halide, on an aromatic ring. Aromatic rings are usually nucleophilic, but some aromatic compoun ...
was invoked when the product distribution was compared between a certain aromatic chloride and an aromatic iodide in reaction with potassium amide. The chloride reaction proceeds through a classical aryne
Arynes and benzynes are highly reactive species derived from an aromatic ring by removal of two substituents. Arynes are examples of didehydroarenes (1,2-didehydroarenes in this case), although 1,3- and 1,4-didehydroarenes are also known. Arynes ar ...
intermediate:
 The
The isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
...
s 1a and 1b form the same aryne 2 which continues to react to the aniline
Aniline is an organic compound with the formula C6 H5 NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile start ...
s 3a and 3b in a 1 to 1.5 ratio. Clear-cut ''cine''-substitution would give a 1:1 ratio, but additional steric and electronic factors come into play as well.
Replacing chlorine
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is ...
by iodine
Iodine is a chemical element with the Symbol (chemistry), symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , ...
in the 1,2,4-trimethylbenzene
1,2,4-Trimethylbenzene, also known as pseudocumene, is an organic compound with the chemical formula CH(CH). Classified as an aromatic hydrocarbon, it is a flammable colorless liquid with a strong odor. It is nearly insoluble in water but soluble ...
moiety drastically changes the product distribution:
 It now resembles ''ipso''-substitution with 1a forming preferentially 3a and 1b forming 3b. Radical scavengers suppress ''ipso''-substitution in favor of ''cine''-substitution and the addition of potassium metal as an electron donor and
It now resembles ''ipso''-substitution with 1a forming preferentially 3a and 1b forming 3b. Radical scavengers suppress ''ipso''-substitution in favor of ''cine''-substitution and the addition of potassium metal as an electron donor and radical initiator
In chemistry, radical initiators are substances that can produce radical species under mild conditions and promote radical reactions. These substances generally possess weak bonds—bonds that have small bond dissociation energies. Radical i ...
does exactly the opposite.''Alkali metal promoted aromatic "nucleophilic" substitution'' Joseph F. Bunnett and Jhong Kook Kim ''J. Am. Chem. Soc.'' 1970, ''92'', 7464 – 7466. ()
See also
*Birch reduction
The Birch reduction is an organic reaction that is used to convert arenes to cyclohexadienes. The reaction is named after the Australian chemist Arthur Birch and involves the organic reduction of aromatic rings in an amine solvent (traditional ...
*Nucleophilic aromatic substitution
A nucleophilic aromatic substitution is a substitution reaction in organic chemistry in which the nucleophile displaces a good leaving group, such as a halide, on an aromatic ring. Aromatic rings are usually nucleophilic, but some aromatic compoun ...
References
{{Organic reactions Free radical reactions Reaction mechanisms