Radioiodine on:
[Wikipedia]
[Google]
[Amazon]
There are 37 known 
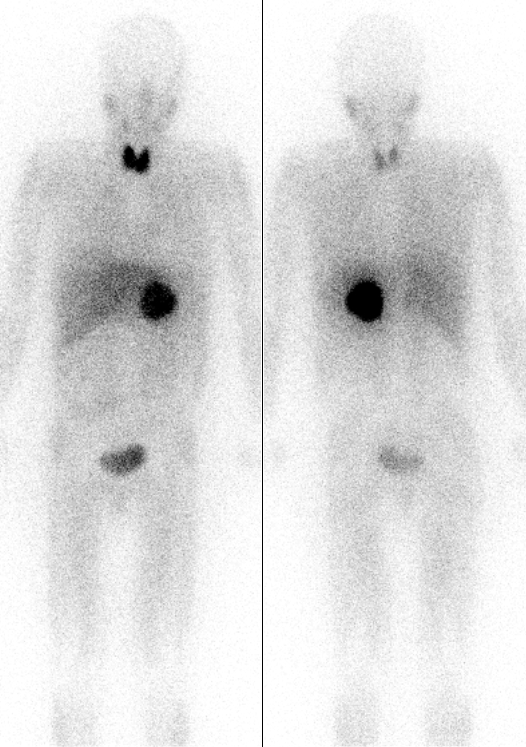 Iodine-131 () is a beta-emitting isotope with a half-life of eight days, and comparatively energetic (190 keV average and 606 keV maximum energy) beta radiation, which penetrates 0.6 to 2.0 mm from the site of uptake. This beta radiation can be used for the destruction of
Iodine-131 () is a beta-emitting isotope with a half-life of eight days, and comparatively energetic (190 keV average and 606 keV maximum energy) beta radiation, which penetrates 0.6 to 2.0 mm from the site of uptake. This beta radiation can be used for the destruction of
Iodine isotopes data from ''The Berkeley Laboratory Isotopes Project's''Iodine-128, Iodine-130, Iodine-132 data from 'Wolframalpha'
{{Authority control Iodine
isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers ( mass num ...
s of iodine
Iodine is a chemical element with the Symbol (chemistry), symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , ...
(53I) from 108I to 144I; all undergo radioactive decay except 127I, which is stable. Iodine is thus a monoisotopic element
A monoisotopic element is an element which has only a single stable isotope (nuclide). There are only 26 elements that have this property. A list is given in a following section.
Stability is experimentally defined for chemical elements, as ther ...
.
Its longest-lived radioactive isotope
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferr ...
, 129I, has a half-life of 15.7 million years, which is far too short for it to exist as a primordial nuclide
In geochemistry, geophysics and nuclear physics, primordial nuclides, also known as primordial isotopes, are nuclides found on Earth that have existed in their current form since before Earth was formed. Primordial nuclides were present in the ...
. Cosmogenic
Cosmogenic nuclides (or cosmogenic isotopes) are rare nuclides (isotopes) created when a high-energy cosmic ray interacts with the nucleus of an ''in situ'' Solar System atom, causing nucleons (protons and neutrons) to be expelled from the atom ...
sources of 129I produce very tiny quantities of it that are too small to affect atomic weight measurements; iodine is thus also a mononuclidic element
A mononuclidic element or monotopic element is one of the 21 chemical elements that is found naturally on Earth essentially as a single nuclide (which may, or may not, be a stable nuclide). This single nuclide will have a characteristic atomic m ...
—one that is found in nature only as a single nuclide. Most 129I derived radioactivity on Earth is man-made, an unwanted long-lived byproduct of early nuclear tests and nuclear fission accidents.
All other iodine radioisotopes have half-lives less than 60 days, and four of these are used as tracers and therapeutic agents in medicine. These are 123I, 124I, 125I, and 131I. All industrial production of radioactive iodine isotopes involves these four useful radionuclides.
The isotope 135I has a half-life less than seven hours, which is too short to be used in biology. Unavoidable ''in situ'' production of this isotope is important in nuclear reactor control, as it decays to 135Xe, the most powerful known neutron absorber
In applications such as nuclear reactors, a neutron poison (also called a neutron absorber or a nuclear poison) is a substance with a large neutron absorption cross-section. In such applications, absorbing neutrons is normally an undesirable eff ...
, and the nuclide
A nuclide (or nucleide, from atomic nucleus, nucleus, also known as nuclear species) is a class of atoms characterized by their number of protons, ''Z'', their number of neutrons, ''N'', and their nuclear energy state.
The word ''nuclide'' was co ...
responsible for the so-called iodine pit
The iodine pit, also called the iodine hole or xenon pit, is a temporary disabling of a nuclear reactor due to buildup of short- lived nuclear poisons in the reactor core. The main isotope responsible is 135Xe, mainly produced by natural decay of ...
phenomenon.
In addition to commercial production, 131I (half-life 8 days) is one of the common radioactive fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release ...
s of nuclear fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radio ...
, and is thus produced inadvertently in very large amounts inside nuclear reactor
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat fr ...
s. Due to its volatility, short half-life, and high abundance in fission products, 131I (along with the short-lived iodine isotope 132I, which is produced from the decay of 132Te with a half-life of 3 days) is responsible for the largest part of radioactive contamination
Radioactive contamination, also called radiological pollution, is the deposition of, or presence of radioactive substances on surfaces or within solids, liquids, or gases (including the human body), where their presence is unintended or undesirab ...
during the first week after accidental environmental contamination from the radioactive waste
Radioactive waste is a type of hazardous waste that contains radioactive material. Radioactive waste is a result of many activities, including nuclear medicine, nuclear research, nuclear power generation, rare-earth mining, and nuclear weapon ...
from a nuclear power plant. Thus highly dosed iodine supplements (usually potassium iodide
Potassium iodide is a chemical compound, medication, and dietary supplement. It is a medication used for treating hyperthyroidism, in radiation emergencies, and for protecting the thyroid gland when certain types of radiopharmaceuticals are u ...
) are given to the populace after nuclear accidents or explosions (and in some cases prior to any such incident as a civil defense
Civil defense ( en, region=gb, civil defence) or civil protection is an effort to protect the citizens of a state (generally non-combatants) from man-made and natural disasters. It uses the principles of emergency operations: prevention, mit ...
mechanism) to reduce the uptake of radioactive iodine compounds by the thyroid
The thyroid, or thyroid gland, is an endocrine gland in vertebrates. In humans it is in the neck and consists of two connected lobes. The lower two thirds of the lobes are connected by a thin band of tissue called the thyroid isthmus. The ...
before the highly radioactive isotopes have had time to decay.
List of isotopes
, - , rowspan=3, 108I , rowspan=3 style="text-align:right" , 53 , rowspan=3 style="text-align:right" , 55 , rowspan=3, 107.94348(39)# , rowspan=3, 36(6) ms , α (90%) , 104Sb , rowspan=3, (1)# , rowspan=3, , rowspan=3, , - , β+ (9%) , 108Te , - , p (1%) , 107Te , - , rowspan=2, 109I , rowspan=2 style="text-align:right" , 53 , rowspan=2 style="text-align:right" , 56 , rowspan=2, 108.93815(11) , rowspan=2, 103(5) µs , p (99.5%) , 108Te , rowspan=2, (5/2+) , rowspan=2, , rowspan=2, , - , α (.5%) , 105Sb , - , rowspan=4, 110I , rowspan=4 style="text-align:right" , 53 , rowspan=4 style="text-align:right" , 57 , rowspan=4, 109.93524(33)# , rowspan=4, 650(20) ms , β+ (70.9%) , 110Te , rowspan=4, 1+# , rowspan=4, , rowspan=4, , - , α (17%) , 106Sb , - , β+, p (11%) , 109Sb , - , β+, α (1.09%) , 106Sn , - , rowspan=2, 111I , rowspan=2 style="text-align:right" , 53 , rowspan=2 style="text-align:right" , 58 , rowspan=2, 110.93028(32)# , rowspan=2, 2.5(2) s , β+ (99.92%) , 111Te , rowspan=2, (5/2+)# , rowspan=2, , rowspan=2, , - , α (.088%) , 107Sb , - , rowspan=4, 112I , rowspan=4 style="text-align:right" , 53 , rowspan=4 style="text-align:right" , 59 , rowspan=4, 111.92797(23)# , rowspan=4, 3.42(11) s , β+ (99.01%) , 112Te , rowspan=4, , rowspan=4, , rowspan=4, , - , β+, p (.88%) , 111Sb , - , β+, α (.104%) , 108Sn , - , α (.0012%) , 108Sb , - , rowspan=3, 113I , rowspan=3 style="text-align:right" , 53 , rowspan=3 style="text-align:right" , 60 , rowspan=3, 112.92364(6) , rowspan=3, 6.6(2) s , β+ (100%) , 113Te , rowspan=3, 5/2+# , rowspan=3, , rowspan=3, , - , α (3.3×10−7%) , 109Sb , - , β+, α , 109Sn , - , rowspan=2, 114I , rowspan=2 style="text-align:right" , 53 , rowspan=2 style="text-align:right" , 61 , rowspan=2, 113.92185(32)# , rowspan=2, 2.1(2) s , β+ , 114Te , rowspan=2, 1+ , rowspan=2, , rowspan=2, , - , β+, p (rare) , 113Sb , - , rowspan=2 style="text-indent:1em" , 114mI , rowspan=2 colspan="3" style="text-indent:2em" , 265.9(5) keV , rowspan=2, 6.2(5) s , β+ (91%) , 114Te , rowspan=2, (7) , rowspan=2, , rowspan=2, , - , IT (9%) , 114I , - , 115I , style="text-align:right" , 53 , style="text-align:right" , 62 , 114.91805(3) , 1.3(2) min , β+ , 115Te , (5/2+)# , , , - , 116I , style="text-align:right" , 53 , style="text-align:right" , 63 , 115.91681(10) , 2.91(15) s , β+ , 116Te , 1+ , , , - , style="text-indent:1em" , 116mI , colspan="3" style="text-indent:2em" , 400(50)# keV , 3.27(16) µs , , , (7−) , , , - , 117I , style="text-align:right" , 53 , style="text-align:right" , 64 , 116.91365(3) , 2.22(4) min , β+ , 117Te , (5/2)+ , , , - , 118I , style="text-align:right" , 53 , style="text-align:right" , 65 , 117.913074(21) , 13.7(5) min , β+ , 118Te , 2− , , , - , rowspan=2 style="text-indent:1em" , 118mI , rowspan=2 colspan="3" style="text-indent:2em" , 190.1(10) keV , rowspan=2, 8.5(5) min , β+ , 118Te , rowspan=2, (7−) , rowspan=2, , rowspan=2, , - , IT (rare) , 118I , - , 119I , style="text-align:right" , 53 , style="text-align:right" , 66 , 118.91007(3) , 19.1(4) min , β+ , 119Te , 5/2+ , , , - , 120I , style="text-align:right" , 53 , style="text-align:right" , 67 , 119.910048(19) , 81.6(2) min , β+ , 120Te , 2− , , , - , style="text-indent:1em" , 120m1I , colspan="3" style="text-indent:2em" , 72.61(9) keV , 228(15) ns , , , (1+, 2+, 3+) , , , - , style="text-indent:1em" , 120m2I , colspan="3" style="text-indent:2em" , 320(15) keV , 53(4) min , β+ , 120Te , (7−) , , , - , 121I , style="text-align:right" , 53 , style="text-align:right" , 68 , 120.907367(11) , 2.12(1) h , β+ , 121Te , 5/2+ , , , - , style="text-indent:1em" , 121mI , colspan="3" style="text-indent:2em" , 2376.9(4) keV , 9.0(15) µs , , , , , , - , 122I , style="text-align:right" , 53 , style="text-align:right" , 69 , 121.907589(6) , 3.63(6) min , β+ , 122Te , 1+ , , , - , 123IHas medical uses , style="text-align:right" , 53 , style="text-align:right" , 70 , 122.905589(4) , 13.2235(19) h , EC , 123Te , 5/2+ , , , - , 124I , style="text-align:right" , 53 , style="text-align:right" , 71 , 123.9062099(25) , 4.1760(3) d , β+ , 124Te , 2− , , , - , 125I , style="text-align:right" , 53 , style="text-align:right" , 72 , 124.9046302(16) , 59.400(10) d , EC , 125Te , 5/2+ , , , - , rowspan=2, 126I , rowspan=2 style="text-align:right" , 53 , rowspan=2 style="text-align:right" , 73 , rowspan=2, 125.905624(4) , rowspan=2, 12.93(5) d , β+ (56.3%) , 126Te , rowspan=2, 2− , rowspan=2, , rowspan=2, , - , β− (43.7%) , 126Xe , - , 127IFission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release ...
, style="text-align:right" , 53
, style="text-align:right" , 74
, 126.904473(4)
, colspan=3 align=center, StableTheoretically capable of spontaneous fission
Spontaneous fission (SF) is a form of radioactive decay that is found only in very heavy chemical elements. The nuclear binding energy of the elements reaches its maximum at an atomic mass number of about 56 (e.g., iron-56); spontaneous breakd ...
, 5/2+
, 1.0000
,
, -
, rowspan=2, 128I
, rowspan=2 style="text-align:right" , 53
, rowspan=2 style="text-align:right" , 75
, rowspan=2, 127.905809(4)
, rowspan=2, 24.99(2) min
, β− (93.1%)
, 128Xe
, rowspan=2, 1+
, rowspan=2,
, rowspan=2,
, -
, β+ (6.9%)
, ''128Te''
, -
, style="text-indent:1em" , 128m1I
, colspan="3" style="text-indent:2em" , 137.850(4) keV
, 845(20) ns
,
,
, 4−
,
,
, -
, style="text-indent:1em" , 128m2I
, colspan="3" style="text-indent:2em" , 167.367(5) keV
, 175(15) ns
,
,
, (6)−
,
,
, -
, 129ICan be used to date certain early events in Solar System history and some use for dating groundwater
, style="text-align:right" , 53
, style="text-align:right" , 76
, 128.904988(3)
, 1.57(4)×107 y
, β−
, 129Xe
, 7/2+
, TraceCosmogenic nuclide
Cosmogenic nuclides (or cosmogenic isotopes) are rare nuclides (isotopes) created when a high-energy cosmic ray interacts with the nucleus of an '' in situ'' Solar System atom, causing nucleons (protons and neutrons) to be expelled from the atom ...
, also found as nuclear contamination
,
, -
, 130I
, style="text-align:right" , 53
, style="text-align:right" , 77
, 129.906674(3)
, 12.36(1) h
, β−
, 130Xe
, 5+
,
,
, -
, rowspan=2 style="text-indent:1em" , 130m1I
, rowspan=2 colspan="3" style="text-indent:2em" , 39.9525(13) keV
, rowspan=2, 8.84(6) min
, IT (84%)
, 130I
, rowspan=2, 2+
, rowspan=2,
, rowspan=2,
, -
, β− (16%)
, 130Xe
, -
, style="text-indent:1em" , 130m2I
, colspan="3" style="text-indent:2em" , 69.5865(7) keV
, 133(7) ns
,
,
, (6)−
,
,
, -
, style="text-indent:1em" , 130m3I
, colspan="3" style="text-indent:2em" , 82.3960(19) keV
, 315(15) ns
,
,
, -
,
,
, -
, style="text-indent:1em" , 130m4I
, colspan="3" style="text-indent:2em" , 85.1099(10) keV
, 254(4) ns
,
,
, (6)−
,
,
, -
, 131I
, style="text-align:right" , 53
, style="text-align:right" , 78
, 130.9061246(12)
, 8.02070(11) d
, β−
, 131Xe
, 7/2+
,
,
, -
, 132I
, style="text-align:right" , 53
, style="text-align:right" , 79
, 131.907997(6)
, 2.295(13) h
, β−
, 132Xe
, 4+
,
,
, -
, rowspan=2 style="text-indent:1em" , 132mI
, rowspan=2 colspan="3" style="text-indent:2em" , 104(12) keV
, rowspan=2, 1.387(15) h
, IT (86%)
, 132I
, rowspan=2, (8−)
, rowspan=2,
, rowspan=2,
, -
, β− (14%)
, 132Xe
, -
, 133I
, style="text-align:right" , 53
, style="text-align:right" , 80
, 132.907797(5)
, 20.8(1) h
, β−
, 133Xe
, 7/2+
,
,
, -
, style="text-indent:1em" , 133m1I
, colspan="3" style="text-indent:2em" , 1634.174(17) keV
, 9(2) s
, IT
, 133I
, (19/2−)
,
,
, -
, style="text-indent:1em" , 133m2I
, colspan="3" style="text-indent:2em" , 1729.160(17) keV
, ~170 ns
,
,
, (15/2−)
,
,
, -
, 134I
, style="text-align:right" , 53
, style="text-align:right" , 81
, 133.909744(9)
, 52.5(2) min
, β−
, 134Xe
, (4)+
,
,
, -
, rowspan=2 style="text-indent:1em" , 134mI
, rowspan=2 colspan="3" style="text-indent:2em" , 316.49(22) keV
, rowspan=2, 3.52(4) min
, IT (97.7%)
, 134I
, rowspan=2, (8)−
, rowspan=2,
, rowspan=2,
, -
, β− (2.3%)
, 134Xe
, -
, 135IProduced as a decay product of 135Te in nuclear reactors, in turn decays to 135Xe, which, if allowed to build up, can shut down reactors due to the iodine pit
The iodine pit, also called the iodine hole or xenon pit, is a temporary disabling of a nuclear reactor due to buildup of short- lived nuclear poisons in the reactor core. The main isotope responsible is 135Xe, mainly produced by natural decay of ...
phenomenon
, style="text-align:right" , 53
, style="text-align:right" , 82
, 134.910048(8)
, 6.57(2) h
, β−
, 135Xe
, 7/2+
,
,
, -
, 136I
, style="text-align:right" , 53
, style="text-align:right" , 83
, 135.91465(5)
, 83.4(10) s
, β−
, ''136Xe''
, (1−)
,
,
, -
, style="text-indent:1em" , 136mI
, colspan="3" style="text-indent:2em" , 650(120) keV
, 46.9(10) s
, β−
, ''136Xe''
, (6−)
,
,
, -
, rowspan=2, 137I
, rowspan=2 style="text-align:right" , 53
, rowspan=2 style="text-align:right" , 84
, rowspan=2, 136.917871(30)
, rowspan=2, 24.13(12) s
, β− (92.86%)
, 137Xe
, rowspan=2, (7/2+)
, rowspan=2,
, rowspan=2,
, -
, β−, n (7.14%)
, ''136Xe''
, -
, rowspan=2, 138I
, rowspan=2 style="text-align:right" , 53
, rowspan=2 style="text-align:right" , 85
, rowspan=2, 137.92235(9)
, rowspan=2, 6.23(3) s
, β− (94.54%)
, 138Xe
, rowspan=2, (2−)
, rowspan=2,
, rowspan=2,
, -
, β−, n (5.46%)
, 137Xe
, -
, rowspan=2, 139I
, rowspan=2 style="text-align:right" , 53
, rowspan=2 style="text-align:right" , 86
, rowspan=2, 138.92610(3)
, rowspan=2, 2.282(10) s
, β− (90%)
, 139Xe
, rowspan=2, 7/2+#
, rowspan=2,
, rowspan=2,
, -
, β−, n (10%)
, 138Xe
, -
, rowspan=2, 140I
, rowspan=2 style="text-align:right" , 53
, rowspan=2 style="text-align:right" , 87
, rowspan=2, 139.93100(21)#
, rowspan=2, 860(40) ms
, β− (90.7%)
, 140Xe
, rowspan=2, (3)(−#)
, rowspan=2,
, rowspan=2,
, -
, β−, n (9.3%)
, 139Xe
, -
, rowspan=2, 141I
, rowspan=2 style="text-align:right" , 53
, rowspan=2 style="text-align:right" , 88
, rowspan=2, 140.93503(21)#
, rowspan=2, 430(20) ms
, β− (78%)
, 141Xe
, rowspan=2, 7/2+#
, rowspan=2,
, rowspan=2,
, -
, β−, n (22%)
, 140Xe
, -
, rowspan=2, 142I
, rowspan=2 style="text-align:right" , 53
, rowspan=2 style="text-align:right" , 89
, rowspan=2, 141.94018(43)#
, rowspan=2, ~200 ms
, β− (75%)
, 142Xe
, rowspan=2, 2−#
, rowspan=2,
, rowspan=2,
, -
, β−, n (25%)
, 141Xe
, -
, 143I
, style="text-align:right" , 53
, style="text-align:right" , 90
, 142.94456(43)#
, 100# ms 300 ns, β−
, 143Xe
, 7/2+#
,
,
, -
, 144I
, style="text-align:right" , 53
, style="text-align:right" , 91
, 143.94999(54)#
, 50# ms 300 ns, β−
, 144Xe
, 1−#
,
,
Notable radioisotopes
Radioisotopes of iodine are called radioactive iodine or radioiodine. Dozens exist, but about a half dozen are the most notable inapplied science
Applied science is the use of the scientific method and knowledge obtained via conclusions from the method to attain practical goals. It includes a broad range of disciplines such as engineering and medicine. Applied science is often contrasted ...
s such as the life sciences and nuclear power, as detailed below. Mentions of radioiodine in health care
Health care or healthcare is the improvement of health via the prevention, diagnosis, treatment, amelioration or cure of disease, illness, injury, and other physical and mental impairments in people. Health care is delivered by health pr ...
contexts refer more often to iodine-131 than to other isotopes.
Of the many isotopes of iodine, only two are typically used in a medical setting: iodine-123 and iodine-131. Since 131I has both a beta and gamma decay mode, it can be used for radiotherapy or for imaging. 123I, which has no beta activity, is more suited for routine nuclear medicine imaging of the thyroid and other medical processes and less damaging internally to the patient. There are some situations in which iodine-124 and iodine-125 are also used in medicine.
Due to preferential uptake of iodine by the thyroid, radioiodine is extensively used in imaging of and, in the case of 131I, destroying dysfunctional thyroid tissues. Other types of tissue selectively take up certain iodine-131-containing tissue-targeting and killing radiopharmaceutical agents (such as MIBG). Iodine-125 is the only other iodine radioisotope used in radiation therapy, but only as an implanted capsule in brachytherapy
Brachytherapy is a form of radiation therapy where a sealed radiation source is placed inside or next to the area requiring treatment. ''Brachy'' is Greek for short. Brachytherapy is commonly used as an effective treatment for cervical, pro ...
, where the isotope never has a chance to be released for chemical interaction with the body's tissues.
Iodine-123 and iodine-125
The gamma-emitting isotopes iodine-123 (half-life 13 hours), and (less commonly) the longer-lived and less energetic iodine-125 (half-life 59 days) are used asnuclear imaging
Nuclear medicine or nucleology is a medical specialty involving the application of radioactive substances in the diagnosis and treatment of disease. Nuclear imaging, in a sense, is "radiology done inside out" because it records radiation emitting ...
tracers to evaluate the anatomic and physiologic function of the thyroid. Abnormal results may be caused by disorders such as Graves' disease
Graves' disease (german: Morbus Basedow), also known as toxic diffuse goiter, is an autoimmune disease that affects the thyroid. It frequently results in and is the most common cause of hyperthyroidism. It also often results in an enlarged thyro ...
or Hashimoto's thyroiditis
Hashimoto's thyroiditis, also known as chronic lymphocytic thyroiditis and Hashimoto's disease, is an autoimmune disease in which the thyroid gland is gradually destroyed. Early on, symptoms may not be noticed. Over time, the thyroid may enlarg ...
. Both isotopes decay by electron capture
Electron capture (K-electron capture, also K-capture, or L-electron capture, L-capture) is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. ...
(EC) to the corresponding tellurium
Tellurium is a chemical element with the symbol Te and atomic number 52. It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur, all three of which are chalcogens. It is occasionall ...
nuclides, but in neither case are these the metastable
In chemistry and physics, metastability denotes an intermediate energetic state within a dynamical system other than the system's state of least energy.
A ball resting in a hollow on a slope is a simple example of metastability. If the ball i ...
nuclides 123mTe and 125mTe (which are of higher energy, and are not produced from radioiodine). Instead, the excited tellurium nuclides decay immediately (half-life too short to detect). Following EC, the excited 123Te from 123I emits a high-speed 127 keV internal conversion
Internal conversion is a non-radioactive, atomic decay process where an excited nucleus interacts electromagnetically with one of the orbital electrons of an atom. This causes the electron to be emitted (ejected) from the atom. Thus, in internal ...
electron (not a beta ray
A beta particle, also called beta ray or beta radiation (symbol β), is a high-energy, high-speed electron or positron emitted by the radioactive decay of an atomic nucleus during the process of beta decay. There are two forms of beta decay, β� ...
) about 13% of the time, but this does little cellular damage due to the nuclide's short half-life and the relatively small fraction of such events. In the remainder of cases, a 159 keV gamma ray is emitted, which is well-suited for gamma imaging.
Excited 125Te resulting from electron capture of 125I also emits a much lower-energy internal conversion electron (35.5 keV), which does relatively little damage due to its low energy, even though its emission is more common. The relatively low-energy gamma from 125I/125Te decay is poorly suited for imaging, but can still be seen, and this longer-lived isotope is necessary in tests that require several days of imaging, for example, fibrinogen scan
Fibrinogen (factor I) is a glycoprotein complex, produced in the liver, that circulates in the blood of all vertebrates. During tissue and vascular injury, it is converted enzymatically by thrombin to fibrin and then to a fibrin-based blood cl ...
imaging to detect blood clots.
Both 123I and 125I emit copious low energy Auger electrons after their decay, but these do not cause serious damage (double-stranded DNA breaks) in cells, unless the nuclide is incorporated into a medication that accumulates in the nucleus, or into DNA (this is never the case is clinical medicine, but it has been seen in experimental animal models).
Iodine-125 is also commonly used by radiation oncologists in low dose rate brachytherapy
Brachytherapy is a form of radiation therapy where a sealed radiation source is placed inside or next to the area requiring treatment. ''Brachy'' is Greek for short. Brachytherapy is commonly used as an effective treatment for cervical, pro ...
in the treatment of cancer at sites other than the thyroid, especially in prostate cancer
Prostate cancer is cancer of the prostate. Prostate cancer is the second most common cancerous tumor worldwide and is the fifth leading cause of cancer-related mortality among men. The prostate is a gland in the male reproductive system that su ...
. When 125I is used therapeutically, it is encapsulated in titanium seeds and implanted in the area of the tumor, where it remains. The low energy of the gamma spectrum in this case limits radiation damage to tissues far from the implanted capsule. Iodine-125, due to its suitable longer half-life and less penetrating gamma spectrum, is also often preferred for laboratory tests that rely on iodine as a tracer that is counted by a gamma counter
A gamma counter is an instrument to measure gamma radiation emitted by a radionuclide. Unlike survey meters, gamma counters are designed to measure small samples of radioactive material, typically with automated measurement and movement of multi ...
, such as in radioimmunoassay
A radioimmunoassay (RIA) is an immunoassay that uses radiolabeled molecules in a stepwise formation of immune complexes. A RIA is a very sensitive in vitro assay technique used to measure concentrations of substances, usually measuring antigen conc ...
ing.
I is used as the radiolabel
A radioactive tracer, radiotracer, or radioactive label is a chemical compound in which one or more atoms have been replaced by a radionuclide so by virtue of its radioactive decay it can be used to explore the mechanism of chemical reactions by tr ...
in investigating which ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's elect ...
s go to which plant pattern recognition receptors (PRRs).
Iodine-124
Iodine-124 is a proton-rich isotope of iodine with a half-life of 4.18 days. Its modes of decay are: 74.4% electron capture, 25.6% positron emission. 124I decays to 124Te. Iodine-124 can be made by numerous nuclear reactions via acyclotron
A cyclotron is a type of particle accelerator invented by Ernest O. Lawrence in 1929–1930 at the University of California, Berkeley, and patented in 1932. Lawrence, Ernest O. ''Method and apparatus for the acceleration of ions'', filed: Jan ...
. The most common starting material used is 124Te.
Iodine-124 as the iodide salt can be used to directly image the thyroid using positron emission tomography
Positron emission tomography (PET) is a functional imaging technique that uses radioactive substances known as radiotracers to visualize and measure changes in metabolic processes, and in other physiological activities including blood flow, ...
(PET). Iodine-124 can also be used as a PET radiotracer
A radioactive tracer, radiotracer, or radioactive label is a chemical compound in which one or more atoms have been replaced by a radionuclide so by virtue of its radioactive decay it can be used to explore the mechanism of chemical reactions by tr ...
with a usefully longer half-life compared with fluorine-18
Fluorine-18 (18F) is a fluorine radioisotope which is an important source of positrons. It has a mass of 18.0009380(6) u and its half-life is 109.771(20) minutes. It decays by positron emission 96% of the time and electron capture 4% of the time ...
. In this use, the nuclide is chemically bonded to a pharmaceutical to form a positron-emitting radiopharmaceutical, and injected into the body, where again it is imaged by PET scan.
Iodine-129
Iodine-129 (129I;half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ...
15.7 million years) is a product of cosmic ray spallation
Cosmic ray spallation, also known as the x-process, is a set of naturally occurring nuclear reactions causing nucleosynthesis; it refers to the formation of chemical elements from the impact of cosmic rays on an object. Cosmic rays are highly ener ...
on various isotopes of xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the ...
in the atmosphere
An atmosphere () is a layer of gas or layers of gases that envelop a planet, and is held in place by the gravity of the planetary body. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A ...
, in cosmic ray
Cosmic rays are high-energy particles or clusters of particles (primarily represented by protons or atomic nuclei) that move through space at nearly the speed of light. They originate from the Sun, from outside of the Solar System in our own ...
muon
A muon ( ; from the Greek letter mu (μ) used to represent it) is an elementary particle similar to the electron, with an electric charge of −1 '' e'' and a spin of , but with a much greater mass. It is classified as a lepton. As w ...
interaction with tellurium-130, and also uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
and plutonium
Plutonium is a radioactive chemical element with the symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exh ...
fission, both in subsurface rocks and nuclear reactors. Artificial nuclear processes, in particular nuclear fuel reprocessing and atmospheric nuclear weapons tests, have now swamped the natural signal for this isotope. Nevertheless, it now serves as a groundwater tracer as indicator of nuclear waste dispersion into the natural environment. In a similar fashion, 129I was used in rainwater studies to track fission products following the Chernobyl disaster
The Chernobyl disaster was a nuclear accident that occurred on 26 April 1986 at the No. 4 reactor in the Chernobyl Nuclear Power Plant, near the city of Pripyat in the north of the Ukrainian SSR in the Soviet Union. It is one of only two n ...
.
In some ways, 129I is similar to 36Cl. It is a soluble halogen, exists mainly as a non-sorbing anion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
, and is produced by cosmogenic, thermonuclear, and in-situ reactions. In hydrologic studies, 129I concentrations are usually reported as the ratio of 129I to total I (which is virtually all 127I). As is the case with 36Cl/Cl, 129I/I ratios in nature are quite small, 10−14 to 10−10 (peak thermonuclear 129I/I during the 1960s and 1970s reached about 10−7). 129I differs from 36Cl in that its half-life is longer (15.7 vs. 0.301 million years), it is highly biophilic, and occurs in multiple ionic forms (commonly, I− and IO3−), which have different chemical behaviors. This makes it fairly easy for 129I to enter the biosphere as it becomes incorporated into vegetation, soil, milk, animal tissue, etc.
Excesses of stable 129Xe in meteorites have been shown to result from decay of "primordial" iodine-129 produced newly by the supernovas that created the dust and gas from which the solar system formed. This isotope has long decayed and is thus referred to as "extinct". Historically, 129I was the first extinct radionuclide
An extinct radionuclide is a radionuclide that was formed by nucleosynthesis before the formation of the Solar System, about 4.6 billion years ago, but has since decayed to virtually zero abundance and is no longer detectable as a primordial nuc ...
to be identified as present in the early Solar System
The Solar System Capitalization of the name varies. The International Astronomical Union, the authoritative body regarding astronomical nomenclature, specifies capitalizing the names of all individual astronomical objects but uses mixed "Solar ...
. Its decay is the basis of the I-Xe iodine-xenon radiometric dating
Radiometric dating, radioactive dating or radioisotope dating is a technique which is used to date materials such as rocks or carbon, in which trace radioactive impurities were selectively incorporated when they were formed. The method compares ...
scheme, which covers the first 85 million years of Solar System
The Solar System Capitalization of the name varies. The International Astronomical Union, the authoritative body regarding astronomical nomenclature, specifies capitalizing the names of all individual astronomical objects but uses mixed "Solar ...
evolution.
Iodine-131
 Iodine-131 () is a beta-emitting isotope with a half-life of eight days, and comparatively energetic (190 keV average and 606 keV maximum energy) beta radiation, which penetrates 0.6 to 2.0 mm from the site of uptake. This beta radiation can be used for the destruction of
Iodine-131 () is a beta-emitting isotope with a half-life of eight days, and comparatively energetic (190 keV average and 606 keV maximum energy) beta radiation, which penetrates 0.6 to 2.0 mm from the site of uptake. This beta radiation can be used for the destruction of thyroid nodule
Thyroid nodules are nodules (raised areas of tissue or fluid) which commonly arise within an otherwise normal thyroid gland. They may be hyperplastic or tumorous, but only a small percentage of thyroid tumors are malignant. Small, asymptomatic ...
s or hyperfunctioning thyroid tissue and for elimination of remaining thyroid tissue after surgery for the treatment of Graves' disease
Graves' disease (german: Morbus Basedow), also known as toxic diffuse goiter, is an autoimmune disease that affects the thyroid. It frequently results in and is the most common cause of hyperthyroidism. It also often results in an enlarged thyro ...
. The purpose of this therapy, which was first explored by Dr. Saul Hertz
Saul Hertz, M.D. (April 20, 1905 – July 28, 1950) was an American physician who devised the medical uses of radioactive iodine. Hertz pioneered the first targeted cancer therapies. Hertz is called the father of the field of theranostics, combini ...
in 1941, is to destroy thyroid tissue that could not be removed surgically. In this procedure, 131I is administered either intravenously or orally following a diagnostic scan. This procedure may also be used, with higher doses of radio-iodine, to treat patients with thyroid cancer
Thyroid cancer is cancer that develops from the tissues of the thyroid gland. It is a disease in which cells grow abnormally and have the potential to spread to other parts of the body. Symptoms can include swelling or a lump in the neck. Ca ...
.
The 131I is taken up into thyroid tissue and concentrated there. The beta particles emitted by the radioisotope destroys the associated thyroid tissue with little damage to surrounding tissues (more than 2.0 mm from the tissues absorbing the iodine). Due to similar destruction, 131I is the iodine radioisotope used in other water-soluble iodine-labeled radiopharmaceutical
Radiopharmaceuticals, or medicinal radiocompounds, are a group of pharmaceutical drugs containing radioactive isotopes. Radiopharmaceuticals can be used as diagnostic and therapeutic agents. Radiopharmaceuticals emit radiation themselves, which ...
s (such as MIBG) used therapeutically to destroy tissues.
The high energy beta radiation (up to 606 keV) from 131I causes it to be the most carcinogenic of the iodine isotopes. It is thought to cause the majority of excess thyroid cancers seen after nuclear fission contamination (such as bomb fallout or severe nuclear reactor accidents like the Chernobyl disaster
The Chernobyl disaster was a nuclear accident that occurred on 26 April 1986 at the No. 4 reactor in the Chernobyl Nuclear Power Plant, near the city of Pripyat in the north of the Ukrainian SSR in the Soviet Union. It is one of only two n ...
) However, these epidemiological effects are seen primarily in children, and treatment of adults and children with therapeutic 131I, and epidemiology of adults exposed to low-dose 131I has not demonstrated carcinogenicity.
Iodine-135
Iodine-135 is an isotope of iodine with a half-life of 6.6 hours. It is an important isotope from the viewpoint ofnuclear reactor physics
Nuclear reactor physics is the field of physics that studies and deals with the applied study and engineering applications of chain reaction to induce a controlled rate of fission in a nuclear reactor for the production of energy.van Dam, H., ...
. It is produced in relatively large amounts as a fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release ...
, and decays to xenon-135
Xenon-135 (135Xe) is an unstable isotope of xenon with a half-life of about 9.2 hours. 135Xe is a fission product of uranium and it is the most powerful known neutron-absorbing nuclear poison (2 million barns; up to 3 million barns under reac ...
, which is a nuclear poison
In applications such as nuclear reactors, a neutron poison (also called a neutron absorber or a nuclear poison) is a substance with a large neutron absorption cross-section. In such applications, absorbing neutrons is normally an undesirable eff ...
with a very large thermal neutron cross section
In nuclear physics, the concept of a neutron cross section is used to express the likelihood of interaction between an incident neutron and a target nucleus. The neutron cross section σ can be defined as the area in cm2 for which the number of ...
, which is a cause of multiple complications in the control of nuclear reactor
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat fr ...
s. The process of buildup of xenon-135
Xenon-135 (135Xe) is an unstable isotope of xenon with a half-life of about 9.2 hours. 135Xe is a fission product of uranium and it is the most powerful known neutron-absorbing nuclear poison (2 million barns; up to 3 million barns under reac ...
from accumulated iodine-135 can temporarily preclude a shut-down reactor from restarting. This is known as xenon poisoning or "falling into an iodine pit
The iodine pit, also called the iodine hole or xenon pit, is a temporary disabling of a nuclear reactor due to buildup of short- lived nuclear poisons in the reactor core. The main isotope responsible is 135Xe, mainly produced by natural decay of ...
".
Iodine-128 and other isotopes
Iodine fission-produced isotopes not discussed above (iodine-128, iodine-130, iodine-132, and iodine-133) have half-lives of several hours or minutes, rendering them almost useless in other applicable areas. Those mentioned are neutron-rich and undergo beta decay to isotopes of xenon. Iodine-128 (half-life 25 minutes) can decay to either tellurium-128 by electron capture or to xenon-128 by beta decay. It has a specific radioactivity of .Nonradioactive iodide (127I) as protection from unwanted radioiodine uptake by the thyroid
Colloquially, radioactive materials can be described as "hot," and non-radioactive materials can be described as "cold." There are instances in which cold iodide is administered to people in order to prevent the uptake of hot iodide by the thyroid gland. For example, blockade of thyroid iodine uptake with potassium iodide is used innuclear medicine
Nuclear medicine or nucleology is a medical specialty involving the application of radioactive substances in the diagnosis and treatment of disease. Nuclear imaging, in a sense, is " radiology done inside out" because it records radiation emi ...
scintigraphy
Scintigraphy (from Latin ''scintilla'', "spark"), also known as a gamma scan, is a diagnostic test in nuclear medicine, where radioisotopes attached to drugs that travel to a specific organ or tissue ( radiopharmaceuticals) are taken internally an ...
and therapy with some radioiodinated compounds that are not targeted to the thyroid, such as iobenguane ( MIBG), which is used to image or treat neural tissue tumors, or iodinated fibrinogen, which is used in fibrinogen scans to investigate clotting. These compounds contain iodine, but not in the iodide form. However, since they may be ultimately metabolized or break down to radioactive iodide, it is common to administer non-radioactive potassium iodide to insure that metabolites of these radiopharmaceuticals is not sequestered by thyroid gland and inadvertently administer a radiological dose to that tissue.
Potassium iodide
Potassium iodide is a chemical compound, medication, and dietary supplement. It is a medication used for treating hyperthyroidism, in radiation emergencies, and for protecting the thyroid gland when certain types of radiopharmaceuticals are u ...
has been distributed to populations exposed to nuclear fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radio ...
accidents such as the Chernobyl disaster
The Chernobyl disaster was a nuclear accident that occurred on 26 April 1986 at the No. 4 reactor in the Chernobyl Nuclear Power Plant, near the city of Pripyat in the north of the Ukrainian SSR in the Soviet Union. It is one of only two n ...
. The iodide solution SSKI, a saturated solution of potassium (K) iodide in water, has been used to block absorption of the radioiodine (it has no effect on other radioisotopes from fission). Tablets containing potassium iodide are now also manufactured and stocked in central disaster sites by some governments for this purpose. In theory, many harmful late-cancer effects of nuclear fallout might be prevented in this way, since an excess of thyroid cancers, presumably due to radioiodine uptake, is the only proven radioisotope contamination effect after a fission accident, or from contamination by fallout from an atomic bomb (prompt radiation from the bomb also causes other cancers, such as leukemias, directly). Taking large amounts of iodide saturates thyroid receptors and prevents uptake of most radioactive iodine-131
Iodine-131 (131I, I-131) is an important radioisotope of iodine discovered by Glenn Seaborg and John Livingood in 1938 at the University of California, Berkeley. It has a radioactive decay half-life of about eight days. It is associated with n ...
that may be present from fission product exposure (although it does not protect from other radioisotopes, nor from any other form of direct radiation). The protective effect of KI lasts approximately 24 hours, so must be dosed daily until a risk of significant exposure to radioiodines from fission products no longer exists.
Iodine-131 (the most common radioiodine contaminant in fallout) also decays relatively rapidly with a half-life of eight days, so that 99.95% of the original radioiodine has vanished after three months.
References
* Isotope masses from: ** * Isotopic compositions and standard atomic masses from: ** ** * Half-life, spin, and isomer data selected from the following sources. ** ** **External links
Iodine isotopes data from ''The Berkeley Laboratory Isotopes Project's''
{{Authority control Iodine
Iodine
Iodine is a chemical element with the Symbol (chemistry), symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , ...
Nuclear safety and security