salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quantitie ...
in the liquid
A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure. As such, it is one of the four fundamental states of matter (the others being solid, gas, a ...
state. In some contexts, the term has been restricted to salts whose melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends ...
is below a specific temperature, such as . While ordinary liquids such as water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a ...
and gasoline
Gasoline (; ) or petrol (; ) (see ) is a transparent, petroleum-derived flammable liquid that is used primarily as a fuel in most spark-ignited internal combustion engines (also known as petrol engines). It consists mostly of organic co ...
are predominantly made of electrically neutral
Electric charge is the physical property of matter that causes charged matter to experience a force when placed in an electromagnetic field. Electric charge can be ''positive'' or ''negative'' (commonly carried by protons and electrons respe ...
molecules
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioche ...
, ionic liquids are largely made of ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
s. These substances are variously called liquid electrolytes, ionic melts, ionic fluids, fused salts, liquid salts, or ionic glasses.
Ionic liquids have many potential applications. They are powerful solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
s and can be used as electrolyte
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dis ...
s. Salts that are liquid at near-ambient temperature are important for electric battery
An electric battery is a source of electric power consisting of one or more electrochemical cells with external connections for powering electrical devices.
When a battery is supplying power, its positive terminal is the cathode and its negati ...
applications, and have been considered as sealants due to their very low vapor pressure
Vapor pressure (or vapour pressure in English-speaking countries other than the US; see spelling differences) or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases ...
.
Any salt that melts without decomposing or vaporizing usually yields an ionic liquid. Sodium chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g ...
(NaCl), for example, melts at into a liquid that consists largely of sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable iso ...
cations () and chloride anions (). Conversely, when an ionic liquid is cooled, it often forms an ionic solid
In chemistry, an ionic compound is a chemical compound composed of ions held together by Coulomb's law, electrostatic forces termed ionic bonding. The compound is neutral overall, but consists of positively charged ions called cations and negativ ...
—which may be either crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macros ...
line or glass
Glass is a non-crystalline, often transparent, amorphous solid that has widespread practical, technological, and decorative use in, for example, window panes, tableware, and optics. Glass is most often formed by rapid cooling (quenching) of ...
y.
The ionic bond is usually stronger than the Van der Waals force
In molecular physics, the van der Waals force is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and th ...
s between the molecules of ordinary liquids. Because of these strong interactions, salts tend to have high lattice energies
In chemistry, the lattice energy is the energy change upon formation of one mole of a crystalline ionic compound from its constituent ions, which are assumed to initially be in the gaseous state. It is a measure of the cohesive forces that bind ...
, manifested in high melting points. Some salts, especially those with organic cations, have low lattice energies and thus are liquid at or below room temperature
Colloquially, "room temperature" is a range of air temperatures that most people prefer for indoor settings. It feels comfortable to a person when they are wearing typical indoor clothing. Human comfort can extend beyond this range depending on ...
. Examples include compounds based on the 1-ethyl-3-methylimidazolium (EMIM) cation and include: EMIM:Cl, EMIMAc (acetate anion), EMIM dicyanamide
Dicyanamide, also known as dicyanamine, is an anion having the formula . It contains two cyanide groups bound to a central nitrogen anion. The chemical is formed by decomposition of 2-cyanoguanidine. It is used extensively as a counterion of org ...
, ()()·, that melts at ; and 1-butyl-3,5-dimethylpyridinium bromide which becomes a glass below .
Low-temperature ionic liquids can be compared to ionic solution
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dis ...
s, liquids that contain both ions and neutral molecules, and in particular to the so-called deep eutectic solvent Deep eutectic solvents or DESs are solutions of Lewis or Brønsted acids and bases which form a eutectic mixture. Deep eutectic solvents are highly tunable through varying the structure or relative ratio of parent components and thus have a wide va ...
s, mixtures of ionic and non-ionic solid substances which have much lower melting points than the pure compounds. Certain mixtures of nitrate salts can have melting points below 100 °C.
The term "ionic liquid" in the general sense was used as early as 1943.
History
The discovery date of the "first" ionic liquid is disputed, along with the identity of its discoverer. Ethanolammonium nitrate (m.p. 52–55 °C) was reported in 1888 by S. Gabriel and J. Weiner. One of the earliest room temperature ionic liquids wasethylammonium nitrate
Ethylammonium nitrate or ethylamine nitrate (EAN) is a salt with formula or ()·. It is an odorless and colorless to slightly yellowish liquid with a melting point of 12 °C. This compound was described by Paul Walden in 1914,
and is believ ...
()· (m.p. 12 °C), reported in 1914 by Paul Walden
Paul Walden ( lv, Pauls Valdens; russian: Павел Иванович Вальден; german: Paul von Walden; 26 July 1863 – 22 January 1957) was a Russian, Latvian and German chemist known for his work in stereochemistry and history of che ...
. In the 1970s and 1980s, ionic liquids based on alkyl-substituted imidazolium and pyridinium cations, with halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluor ...
or tetrahalogenoaluminate anions, were developed as potential electrolytes in batteries.
For the imidazolium halogenoaluminate salts, their physical properties—such as viscosity
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water.
Viscosity quantifies the inte ...
, melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends ...
, and acidity—could be adjusted by changing the alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloalk ...
substituent
A substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule. (In organic chemistry and biochemistry, the terms ''substituent'' and ''functional group'', as well as ''side ...
s and the imidazolium/pyridinium and halide/halogenoaluminate ratios. Two major drawbacks for some applications were moisture sensitivity and acidity or basicity. In 1992, Wilkes and Zawarotko obtained ionic liquids with 'neutral' weakly coordinating anions such as hexafluorophosphate
Hexafluorophosphate is an anion with chemical formula of . It is an octahedral species that imparts no color to its salts. is isoelectronic with sulfur hexafluoride, , and the hexafluorosilicate dianion, , and hexafluoroantimonate . In this anio ...
() and tetrafluoroborate (), allowing a much wider range of applications.
Characteristics
IL's are typically colorless viscous liquids. They are often moderate to poor conductors of electricity, non-ionizing. They exhibit lowvapor pressure
Vapor pressure (or vapour pressure in English-speaking countries other than the US; see spelling differences) or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases ...
. Many have low combustibility and are thermally stable.
The solubility properties of ILs are diverse. Saturated aliphatic
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated, like hexane, or ...
compounds are generally only sparingly soluble in ionic liquids, whereas alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
s show somewhat greater solubility, and aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
s often completely miscible. Solubility differences can be exploited in biphasic catalysis, such as hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a Catalysis, catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or S ...
and hydrocarbonylation
Carbonylation refers to Chemical reaction, reactions that introduce carbon monoxide into Organic compound, organic and inorganic compound, inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely use ...
processes, allowing for relatively easy separation of products and/or unreacted substrate(s). Gas solubility follows the same trend, with carbon dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transpar ...
gas showing good solubility in many ionic liquids. Carbon monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simple ...
is less soluble in ionic liquids than in many popular organic solvents, and hydrogen is only slightly soluble (similar to the solubility in water) and may vary relatively little between the more common ionic liquids.
Many classes of chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
s, The miscibility of ionic liquids with water or organic solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
s varies with side chain lengths on the cation and with choice of anion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
. They can be functionalized to act as acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequ ...
s, bases, or ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electr ...
s, and are precursors salts in the preparation of stable carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
The term "carbene" ma ...
s. Because of their distinctive properties, ionic liquids have been investigated for many applications.
 Some ionic liquids can be
Some ionic liquids can be distill
Distillation, or classical distillation, is the process of separating the components or substances from a liquid mixture by using selective boiling and condensation, usually inside an apparatus known as a still. Dry distillation is the heating ...
ed under vacuum conditions at temperatures near 300 °C. The vapor is not made up of separated ions, but consists of ion pairs.
ILs have a wide liquid range. Some ILs do not freeze down to very low temperatures (even −150 °C), The glass transition temperature
The glass–liquid transition, or glass transition, is the gradual and reversible transition in amorphous materials (or in amorphous regions within semicrystalline materials) from a hard and relatively brittle "glassy" state into a viscous or rubb ...
was detected below −100 °C in the case of N-methyl-N-alkylpyrrolidinium cations fluorosulfonyl-trifluoromethanesulfonylimide (FTFSI). Low-temperature ionic liquids (below 130 K) have been proposed as the fluid base for an extremely large diameter spinning liquid-mirror telescope to be based on the Moon.
Water is a common impurity in ionic liquids, as it can be absorbed from the atmosphere and influences the transport properties of RTILs, even at relatively low concentrations.
Varieties
Cations
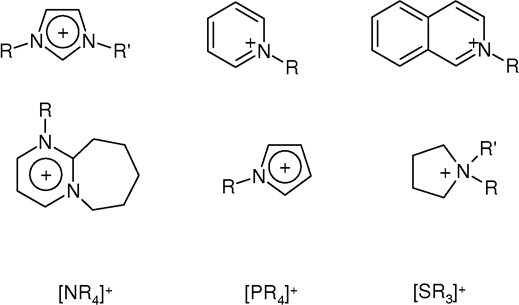
Room-temperature ionic liquids (RTILs) are dominated by salts derived from 1-methylimidazole, i.e., 1-alkyl-3-methylimidazolium. Examples include 1-ethyl-3-methyl- (EMIM), 1-butyl-3-methyl- (BMIM), 1-octyl-3 methyl (OMIM), 1-decyl-3-methyl-(DMIM), 1-dodecyl-3-methyl- docecylMIM). Other imidazolium cations are 1-butyl-2,3-dimethylimidazolium (BMMIM or DBMIM) and 1,3-di(N,N-dimethylaminoethyl)-2-methylimidazolium (DAMI). Other N-heterocyclic cations are derived frompyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a d ...
: 4-methyl-N-butyl-pyridinium (MBPy) and N-octylpyridinium (C8Py). Conventional quaternary ammonium cations also form ILs, e.g. tetraethylammonium (TEA) and tetrabutylammonium (TBA).
Anions
Typical anions in ionic liquids include the following: tetrafluoroborate (BF4), hexafluorophosphate (PF6), bis-trifluoromethanesulfonimide (NTf2), trifluoromethanesulfonate (OTf), dicyanamide (N(CN)2), hydrogen sulphate (HSO4), and ethyl sulphate (EtOSO3). Magnetic ionic liquids can be synthesized by incorporating paramagnetic anions, illustrated by1-butyl-3-methylimidazolium tetrachloroferrate
1-Butyl-3-methylimidazolium tetrachloroferrate is a magnetic ionic liquid. It can be obtained from 1-butyl-3-methylimidazolium chloride and ferric chloride. It has quite low water solubility.
Due to the presence of the high spin FeCl4 anion, the ...
.
Specialized IL's
Protic ionic liquid A protic ionic liquid is an ionic liquid that is formed via proton transfer from a Brønsted acid to a Brønsted base. Unlike many other types of ionic liquids, which are formed through a series of synthesis steps, protic ionic liquids are easier ...
s are formed via a proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
transfer from an acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequ ...
to a base. In contrast to other ionic liquids, which generally are formed through a sequence of synthesis
Synthesis or synthesize may refer to:
Science Chemistry and biochemistry
*Chemical synthesis, the execution of chemical reactions to form a more complex molecule from chemical precursors
** Organic synthesis, the chemical synthesis of organ ...
steps, protic ionic liquids can be created more easily by simply mixing the acid and base.
Phosphonium
In polyatomic cations with the chemical formula (where R is a hydrogen or an alkyl, aryl, or halide group). These cations have tetrahedral structures. The salts are generally colorless or take the color of the anions.
Types of phosphonium ...
cations (R4P+) are less common but offer some advantageous properties. Some examples of phosphonium cations are trihexyl(tetradecyl)phosphonium (P6,6,6,14) and tributyl(tetradecyl)phosphonium (P4,4,4,14).
Poly(ionic liquid)s
Polymerized ionic liquids, poly(ionic liquid)s or polymeric ionic liquids, all abbreviated as PIL is the polymeric form of ionic liquids. They have half of the ionicity of ionic liquids since one ion is fixed as the polymer moiety to form a polymeric chain. PILs have a similar range of applications, comparable with those of ionic liquids but the polymer architecture provides a better chance for controlling the ionic conductivity. They have extended the applications of ionic liquids for designing smart materials or solid electrolytes.Commercial applications
Many applications have been considered, but few have been commercialized. ILs are used in the production of gasoline by catalyzingalkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting ...
.
 An IL based on tetraalkyl
An IL based on tetraalkylphosphonium
In polyatomic cations with the chemical formula (where R is a hydrogen or an alkyl, aryl, or halide group). These cations have tetrahedral structures. The salts are generally colorless or take the color of the anions.
Types of phosphonium ...
iodide is a solvent for tributyltin iodide, which functions as a catalyst to rearrange the monoepoxide of butadiene
1,3-Butadiene () is the organic compound with the formula (CH2=CH)2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two viny ...
. This process was commercialized as a route to 2,5-dihydrofuran
2,5-Dihydrofuran is an organic compound classified as a monounsaturated derivative of furan. It is a colorless, volatile liquid. It can be produced by the rearrangement of the epoxide of butadiene.G. Wytze Meindersma, Matthias Maase and André ...
, but later discontinued.
Potential applications
Catalysis
ILs improve the catalytic performance of palladium nanoparticles. Furthermore, ionic liquids can be used pre-catalysts for chemical transformations. In this regard dialkylimidazoliums such asMIM
MIM or Mim may refer to:
Places
* Mim, Ahafo, Ghana
* Mim Lake, Ghana
* Mim Bour, or Mim Mountains, Ghana
Education
* Master of Management, a post-graduate master's degree
* Master of Information Management, an interdisciplinary degree progra ...
c have been used in the combination with a base to generate N-heterocyclic carbenes
A persistent carbene (also known as stable carbene) is a type of carbene demonstrating particular stability. The best-known examples and by far largest subgroup are the ''N''-heterocyclic carbenes (NHC) (sometimes called Arduengo carbenes), for ex ...
(NHCs). These imidazolium based NHCs are known to catalyse a number transformations such as the Benzoin condensation
The benzoin addition is an addition reaction involving two aldehydes. The reaction generally occurs between aromatic aldehydes or glyoxals, and results in formation of an acyloin. In the classic example, benzaldehyde is converted to benzoin.
Th ...
and the OTHO reaction.
Pharmaceuticals
Recognizing that approximately 50% of commercial pharmaceuticals are salts, ionic liquid forms of a number of pharmaceuticals have been investigated. Combining a pharmaceutically active cation with a pharmaceutically active anion leads to a Dual Active ionic liquid in which the actions of two drugs are combined. ILs can extract specific compounds from plants for pharmaceutical, nutritional and cosmetic applications, such as the antimalarial drugartemisinin
Artemisinin () and its semisynthetic derivatives are a group of drugs used in the treatment of malaria due to ''Plasmodium falciparum''. It was discovered in 1972 by Tu Youyou, who shared the 2015 Nobel Prize in Physiology or Medicine for her dis ...
from the plant ''Artemisia annua
''Artemisia annua'', also known as sweet wormwood, sweet annie, sweet sagewort, annual mugwort or annual wormwood (), is a common type of wormwood native to temperate Asia, but naturalized in many countries including scattered parts of North Am ...
''.
Biopolymer processing
The dissolution ofcellulose
Cellulose is an organic compound with the formula , a polysaccharide consisting of a linear chain of several hundred to many thousands of β(1→4) linked D-glucose units. Cellulose is an important structural component of the primary cell wall ...
by ILs has attracted interest. A patent application from 1930 showed that 1-alkylpyridinium chlorides dissolve cellulose. Following in the footsteps of the lyocell
Lyocell, originally trademarked in 1982 as Tencel, is a form of regenerated cellulose. It consists of cellulose fibers, made by dissolving pulp and then reconstituting it by dry jet-wet spinning. The fiber is used to make textiles for clothing a ...
process, which uses hydrated N-Methylmorpholine N-oxide
''N''-Methylmorpholine ''N''-oxide (more correctly 4-methylmorpholine 4-oxide), NMO or NMMO is an organic compound. This heterocyclic amine oxide and morpholine derivative is used in organic chemistry as a co-oxidant and sacrificial catalyst in ...
as a solvent for pulp and paper. The "valorization" of cellulose, i.e. its conversion to more valuable chemicals, has been achieved by the use of ionic liquids. Representative products are glucose esters, sorbitol
Sorbitol (), less commonly known as glucitol (), is a sugar alcohol with a sweet taste which the human body metabolizes slowly. It can be obtained by reduction of glucose, which changes the converted aldehyde group (−CHO) to a primary alcohol g ...
, and alkylgycosides. IL 1-butyl-3-methylimidazolium chloride dissolves freeze dried banana
A banana is an elongated, edible fruit – botanically a berry – produced by several kinds of large herbaceous flowering plants in the genus ''Musa''. In some countries, bananas used for cooking may be called "plantains", distinguis ...
pulp and with an additional 15% dimethyl sulfoxide
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula ( CH3)2. This colorless liquid is the sulfoxide most widely used commercially. It is an important polar aprotic solvent that dissolves both polar and nonpolar compounds a ...
, lends itself to Carbon-13 NMR Carbon-13 (C13) nuclear magnetic resonance (most commonly known as carbon-13 NMR spectroscopy or 13C NMR spectroscopy or sometimes simply referred to as carbon NMR) is the application of nuclear magnetic resonance (NMR) spectroscopy to carbon. It is ...
analysis. In this way the entire complex of starch
Starch or amylum is a polymeric carbohydrate consisting of numerous glucose units joined by glycosidic bonds. This polysaccharide is produced by most green plants for energy storage. Worldwide, it is the most common carbohydrate in human diets ...
, sucrose
Sucrose, a disaccharide, is a sugar composed of glucose and fructose subunits. It is produced naturally in plants and is the main constituent of white sugar. It has the molecular formula .
For human consumption, sucrose is extracted and refined ...
, glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using ...
, and fructose
Fructose, or fruit sugar, is a Ketose, ketonic monosaccharide, simple sugar found in many plants, where it is often bonded to glucose to form the disaccharide sucrose. It is one of the three dietary monosaccharides, along with glucose and galacto ...
can be monitored as a function of banana ripening.
Beyond cellulose, ILs have also shown potential in the dissolution, extraction, purification, processing and modification of other biopolymer
Biopolymers are natural polymers produced by the cells of living organisms. Like other polymers, biopolymers consist of monomeric units that are covalently bonded in chains to form larger molecules. There are three main classes of biopolymers, cl ...
s such as chitin
Chitin ( C8 H13 O5 N)n ( ) is a long-chain polymer of ''N''-acetylglucosamine, an amide derivative of glucose. Chitin is probably the second most abundant polysaccharide in nature (behind only cellulose); an estimated 1 billion tons of chit ...
/ chitosan, starch
Starch or amylum is a polymeric carbohydrate consisting of numerous glucose units joined by glycosidic bonds. This polysaccharide is produced by most green plants for energy storage. Worldwide, it is the most common carbohydrate in human diets ...
, alginate, collagen, gelatin
Gelatin or gelatine (from la, gelatus meaning "stiff" or "frozen") is a translucent, colorless, flavorless food ingredient, commonly derived from collagen taken from animal body parts. It is brittle when dry and rubbery when moist. It may also ...
, keratin
Keratin () is one of a family of structural fibrous proteins also known as ''scleroproteins''. Alpha-keratin (α-keratin) is a type of keratin found in vertebrates. It is the key structural material making up scales, hair, nails, feathers, ho ...
, and fibroin. For example, ILs allow for the preparation of biopolymer materials in different forms (e.g. sponges, films, microparticles, nanoparticles, and aerogels) and better biopolymer chemical reactions, leading to biopolymer-based drug/gene-delivery carriers. Moreover, ILs enable the synthesis of chemically modified starches with high efficiency and degrees of substitution (DS) and the development of various starch-based materials such as thermoplastic starch, composite films, solid polymer electrolytes, nanoparticles and drug carriers.
Nuclear fuel reprocessing
The IL 1-butyl-3-methylimidazolium chloride has been investigated for the recovery ofuranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
and other metals from spent nuclear fuel
Nuclear fuel is material used in nuclear power stations to produce heat to power turbines. Heat is created when nuclear fuel undergoes nuclear fission.
Most nuclear fuels contain heavy fissile actinide elements that are capable of undergoing ...
and other sources.
Solar thermal energy
ILs are potential heat transfer and storage media in solar thermal energy systems. Concentrating solar thermal facilities such asparabolic trough
A parabolic trough is a type of solar thermal collector that is straight in one dimension and curved as a parabola in the other two, lined with a polished metal mirror. The sunlight which enters the mirror parallel to its plane of symmetry is foc ...
s and solar power tower
A solar power tower, also known as 'central tower' power plant or 'heliostat' power plant, is a type of solar furnace using a tower to receive focused sunlight. It uses an array of flat, movable mirrors (called heliostats) to focus the sun's ra ...
s focus the sun's energy onto a receiver, which can generate temperatures of around . This heat can then generate electricity in a steam or other cycle. For buffering during cloudy periods or to enable generation overnight, energy can be stored by heating an intermediate fluid. Although nitrate salts have been the medium of choice since the early 1980s, they freeze at and thus require heating to prevent solidification. Ionic liquids such as 4mim] have more favorable liquid-phase temperature ranges (-75 to 459 °C) and could therefore be excellent liquid thermal storage media and heat transfer fluids.
Waste recycling
ILs can aid the recycling of synthetic goods, plastics, and metals. They offer the specificity required to separate similar compounds from each other, such as separatingpolymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
s in plastic waste
Plastic pollution is the accumulation of plastic objects and particles (e.g. plastic bottles, bags and microbeads) in the Earth's environment that adversely affects humans, wildlife and their habitat. Plastics that act as pollutants are catego ...
streams. This has been achieved using lower temperature extraction processes than current approaches and could help avoid incinerating plastics or dumping them in landfill.
Batteries
ILs can replace water as the electrolyte in metal-air batteries. ILs are attractive because of their low vapor pressure. Furthermore, ILs have anelectrochemical window The electrochemical window (EW) of a substance is the electrode electric potential range between which the substance is neither oxidized nor reduced. The EW is one of the most important characteristics to be identified for solvents and electrolyte ...
of up to six volts (versus 1.23 for water) supporting more energy-dense metals. Energy densities from 900 to 1600 watt-hours per kilogram appear possible.
Dispersing agent
ILs can act asdispersing agent
A dispersant or a dispersing agent is a substance, typically a surfactant, that is added to a suspension of solid or liquid particles in a liquid (such as a colloid or emulsion) to improve the separation of the particles and to prevent their settl ...
s in paint
Paint is any pigmented liquid, liquefiable, or solid mastic composition that, after application to a substrate in a thin layer, converts to a solid film. It is most commonly used to protect, color, or provide texture. Paint can be made in many ...
s to enhance finish, appearance and drying properties. ILs are used for dispersing nanomaterial
*
Nanomaterials describe, in principle, materials of which a single unit is sized (in at least one dimension) between 1 and 100 nm (the usual definition of nanoscale).
Nanomaterials research takes a materials science-based approach to nan ...
s at IOLITEC.
Carbon capture
ILs andamine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituen ...
s have been investigated for capturing carbon dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transpar ...
and purifying natural gas.
Tribology
Some ionic liquids have been shown to reduce friction and wear in basic tribological testing, and their polar nature makes them candidatelubricant
A lubricant (sometimes shortened to lube) is a substance that helps to reduce friction between surfaces in mutual contact, which ultimately reduces the heat generated when the surfaces move. It may also have the function of transmitting forces, t ...
s for tribotronic applications. While the comparatively high cost of ionic liquids currently prevents their use as neat lubricants, adding ionic liquids in concentrations as low as 0.5 wt% may significantly alter the lubricating performance of conventional base oils. Thus, the current focus of research is on using ionic liquids as additives to lubricating oils, often with the motivation to replace widely used, ecologically harmful lubricant additives. However, the claimed ecological advantage of ionic liquids has been questioned repeatedly and is yet to be demonstrated from a lifecycle
Life cycle, life-cycle, or lifecycle may refer to:
Science and academia
* Biological life cycle, the sequence of life stages that an organism undergoes from birth to reproduction ending with the production of the offspring
*Life-cycle hypothesis ...
perspective.
Safety
Ionic liquids' low volatility effectively eliminates a major pathway for environmental release and contamination. Ionic liquids' aquatic toxicity is as severe as or more so than many current solvents.Ultrasound
Ultrasound is sound waves with frequency, frequencies higher than the upper audible limit of human hearing range, hearing. Ultrasound is not different from "normal" (audible) sound in its physical properties, except that humans cannot hea ...
can degrade solutions of imidazolium-based ionic liquids with hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%� ...
and acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component ...
to relatively innocuous compounds.
Despite low vapor pressure
Vapor pressure (or vapour pressure in English-speaking countries other than the US; see spelling differences) or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases ...
many ionic liquids are combustible.
See also
*MDynaMix
Molecular Dynamics of Mixtures (MDynaMix) is a computer software package for general purpose molecular dynamics to simulate mixtures of molecules, interacting by AMBER- and CHARMM-like Force field (chemistry), force fields in periodic boundary cond ...
software for ionic liquids simulations
* 1-Butyl-3-methylimidazolium hexafluorophosphate
1-Butyl-3-methylimidazolium hexafluorophosphate, also known as BMIM-PF6, is a viscous, colourless, hydrophobic and non-water-soluble ionic liquid with a melting point of -8 °C. Together with 1-butyl-3-methylimidazolium tetrafluoroborate, BMI ...
(BMIM-PF6) for an often encountered ionic liquid
* Trioctylmethylammonium bis(trifluoromethyl-sulfonyl)imide
Trioctylmethylammonium bis(trifluoromethylsulfonyl)imide is an ionic liquid that is produced by Solvent Innovation, now part of Merck KGaA, EMD Chemicals.
References
Ionic liquids
Trifluoromethyl compounds
{{Organic-compound-stub ...
* Aza-Baylis–Hillman reaction
The aza-Baylis–Hillman reaction or aza-BH reaction in organic chemistry is a variation of the Baylis–Hillman reaction and describes the reaction of an electron deficient alkene, usually an α,β-unsaturated carbonyl compound, with an imine in ...
for the use of a chiral ionic liquid in asymmetric synthesis
Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molecul ...
.
* Ionic liquids in carbon capture The use of ionic liquids in carbon capture is a potential application of ionic liquids as absorbents for use in carbon capture and sequestration. Ionic liquids, which are salts that exist as liquids near room temperature, are polar, nonvolatile m ...
* NanoFlowcell
nanoFlowcell Holdings plc is a Swiss flow cell battery research and development company.
nanoFlowcell claims to have developed the first flow battery small enough to be used in electric cars. Its battery, also branded nanoFlowcell, was first p ...
which uses ionic liquid in its car batteries
* Ioliomics
Ioliomics (from a portmanteau of ions and liquids) is the study of ions in liquids (or liquid phases) and stipulated with fundamental differences of ionic interactions. Ioliomics covers a broad research area concerning structure, properties and ...
, or studies of ions in liquids
References
External links
Ionic Liquids Biological Effects Database
, free database on toxicology and ecotoxicology of ionic liquids
Corresponding states for ionic fluids
{{Authority control Ions