Resting membrane potential on:
[Wikipedia]
[Google]
[Amazon]
 The relatively static
The relatively static
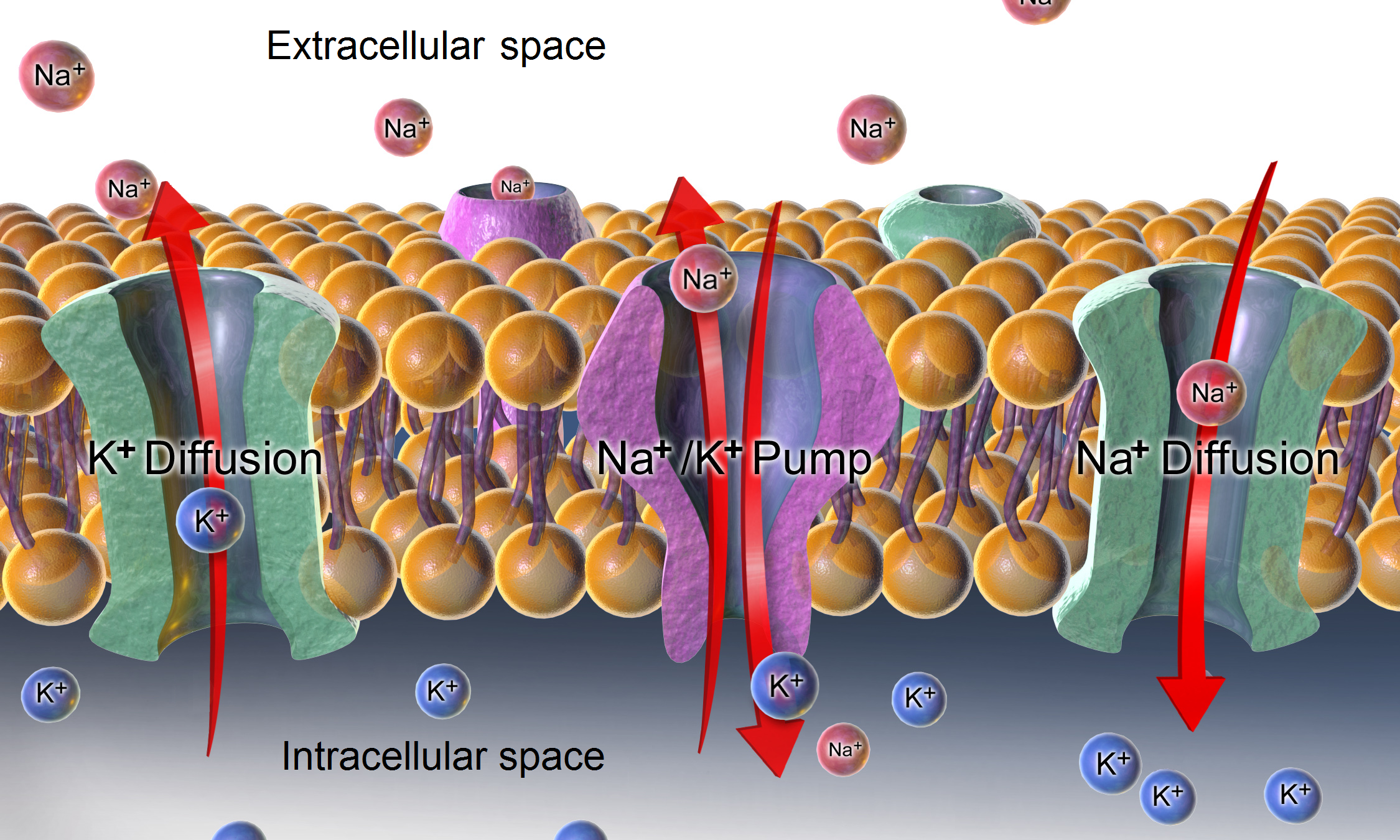
Because the membrane permeability for potassium is much higher than that for other ions, and because of the strong chemical gradient for potassium, potassium ions flow from the cytosol out to the extracellular space carrying out positive charge, until their movement is balanced by build-up of negative charge on the inner surface of the membrane. Again, because of the high relative permeability for potassium, the resulting membrane potential is almost always close to the potassium reversal potential. But in order for this process to occur, a concentration gradient of potassium ions must first be set up. This work is done by the ion pumps/transporters and/or exchangers and generally is powered by ATP. In the case of the resting membrane potential across an animal cell's
Note that even though the membrane potential at 0 mV is stable, it is not an equilibrium condition because neither of the contributing ions is in equilibrium. Ions diffuse down their electrochemical gradients through ion channels, but the membrane potential is upheld by continual K+ influx and Na+ efflux via ion transporters. Such situation with similar permeabilities for counter-acting ions, like potassium and sodium in animal cells, can be extremely costly for the cell if these permeabilities are relatively large, as it takes a lot of ATP energy to pump the ions back. Because no real cell can afford such equal and large ionic permeabilities at rest, resting potential of animal cells is determined by predominant high permeability to potassium and adjusted to the required value by modulating sodium and chloride permeabilities and gradients. In a healthy animal cell Na+ permeability is about 5% of the K+ permeability or even less, whereas the respective reversal potentials are +60 mV for sodium (''E''Na)and −80 mV for potassium (''E''K). Thus the membrane potential will not be right at ''E''K, but rather depolarized from ''E''K by an amount of approximately 5% of the 140 mV difference between ''E''K and ''E''Na. Thus, the cell's resting potential will be about −73 mV. In a more formal notation, the membrane potential is the
example
of an
Neuroscience
- online textbook by Purves, et al.
Basic Neurochemistry
Molecular, Cellular, and Medical Aspects by Siegel, et al. *
Resting Membrane Potential
- Online lecture notes on the resting membrane potential
- Online interactive tutorial {{DEFAULTSORT:Resting Potential Neurophysiology Membrane biology Potentials
 The relatively static
The relatively static membrane potential
Membrane potential (also transmembrane potential or membrane voltage) is the difference in electric potential between the interior and the exterior of a biological cell. It equals the interior potential minus the exterior potential. This is th ...
of quiescent cells is called the resting membrane potential (or resting voltage), as opposed to the specific dynamic electrochemical phenomena called action potential
An action potential (also known as a nerve impulse or "spike" when in a neuron) is a series of quick changes in voltage across a cell membrane. An action potential occurs when the membrane potential of a specific Cell (biology), cell rapidly ri ...
and graded membrane potential
Membrane potential (also transmembrane potential or membrane voltage) is the difference in electric potential between the interior and the exterior of a biological cell. It equals the interior potential minus the exterior potential. This is th ...
. The resting membrane potential has a value of approximately −70 mV or −0.07 V.
Apart from the latter two, which occur in excitable cells (neuron
A neuron (American English), neurone (British English), or nerve cell, is an membrane potential#Cell excitability, excitable cell (biology), cell that fires electric signals called action potentials across a neural network (biology), neural net ...
s, muscle
Muscle is a soft tissue, one of the four basic types of animal tissue. There are three types of muscle tissue in vertebrates: skeletal muscle, cardiac muscle, and smooth muscle. Muscle tissue gives skeletal muscles the ability to muscle contra ...
s, and some secretory cells in gland
A gland is a Cell (biology), cell or an Organ (biology), organ in an animal's body that produces and secretes different substances that the organism needs, either into the bloodstream or into a body cavity or outer surface. A gland may also funct ...
s), membrane voltage in the majority of non-excitable cells can also undergo changes in response to environmental or intracellular stimuli. The resting potential exists due to the differences in membrane permeabilities for potassium
Potassium is a chemical element; it has Symbol (chemistry), symbol K (from Neo-Latin ) and atomic number19. It is a silvery white metal that is soft enough to easily cut with a knife. Potassium metal reacts rapidly with atmospheric oxygen to ...
, sodium
Sodium is a chemical element; it has Symbol (chemistry), symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 element, group 1 of the peri ...
, calcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to it ...
, and chloride
The term chloride refers to a compound or molecule that contains either a chlorine anion (), which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond (). The pr ...
ions, which in turn result from functional activity of various ion channel
Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by Gating (electrophysiol ...
s, ion transporters, and exchangers. Conventionally, resting membrane potential can be defined as a relatively stable, ground value of transmembrane voltage in animal and plant cells.Because the membrane permeability for potassium is much higher than that for other ions, and because of the strong chemical gradient for potassium, potassium ions flow from the cytosol out to the extracellular space carrying out positive charge, until their movement is balanced by build-up of negative charge on the inner surface of the membrane. Again, because of the high relative permeability for potassium, the resulting membrane potential is almost always close to the potassium reversal potential. But in order for this process to occur, a concentration gradient of potassium ions must first be set up. This work is done by the ion pumps/transporters and/or exchangers and generally is powered by ATP. In the case of the resting membrane potential across an animal cell's
plasma membrane
The cell membrane (also known as the plasma membrane or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of a cell from the outside environment (the extr ...
, potassium (and sodium) gradients are established by the Na+/K+-ATPase (sodium-potassium pump) which transports 2 potassium ions inside and 3 sodium ions outside at the cost of 1 ATP molecule. In other cases, for example, a membrane potential may be established by acidification of the inside of a membranous compartment (such as the proton pump that generates membrane potential across synaptic vesicle
In a neuron, synaptic vesicles (or neurotransmitter vesicles) store various neurotransmitters that are exocytosis, released at the chemical synapse, synapse. The release is regulated by a voltage-dependent calcium channel. Vesicle (biology), Ves ...
membranes).
Electroneutrality
In most quantitative treatments of membrane potential, such as the derivation of Goldman equation, electroneutrality is assumed; that is, that there is no measurable charge excess on either side of the membrane. So, although there is an electric potential across the membrane due to charge separation, there is no actual measurable difference in the global concentration of positive and negative ions across the membrane (as it is estimated below), that is, there is no actual measurable charge excess on either side. That occurs because the effect of charge on electrochemical potential is hugely greater than the effect of concentration so an undetectable change in concentration creates a great change in electric potential.Generation of the resting potential
Cell membranes are typically permeable to only a subset of ions. These usually include potassium ions, chloride ions, bicarbonate ions, and others. To simplify the description of the ionic basis of the resting membrane potential, it is most useful to consider only one ionic species at first, and consider the others later. Since trans-plasma-membrane potentials are almost always determined primarily by potassium permeability, that is where to start. *Panel 1 of the diagram shows a diagrammatic representation of a simple cell where a concentration gradient has already been established. This panel is drawn as if the membrane has no permeability to any ion. There is no membrane potential because despite there being a concentration gradient for potassium, there is no net charge imbalance across the membrane. If the membrane were to become permeable to a type of ion that is more concentrated on one side of the membrane, then that ion would contribute to membrane voltage because the permeant ions would move across the membrane with net movement of that ion type down the concentration gradient. There would be net movement from the side of the membrane with a higher concentration of the ion to the side with lower concentration. Such a movement of one ion across the membrane would result in a net imbalance of charge across the membrane and a membrane potential. This is a common mechanism by which many cells establish a membrane potential. *In panel 2 of the diagram, the cell membrane has been made permeable to potassium ions, but not the anions (An−) inside the cell. These anions are mostly contributed by protein. There is energy stored in the potassium ion concentration gradient that can be converted into an electrical gradient when potassium (K+) ions move out of the cell. Note that potassium ions can move across the membrane in both directions but by the purely statistical process that arises from the higher concentration of potassium ions inside the cell, there will be more potassium ions moving out of the cell. Because there is a higher concentration of potassium ions inside the cells, their random molecular motion is more likely to encounter the permeability pore (ion channel
Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by Gating (electrophysiol ...
) that is the case for the potassium ions that are outside and at a lower concentration. An internal K+ is simply "more likely" to leave the cell than an extracellular K+ is to enter it. It is a matter of diffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical p ...
doing work by dissipating the concentration gradient. As potassium leaves the cell, it is leaving behind the anions. Therefore, a charge separation is developing as K+ leaves the cell. This charge separation creates a transmembrane voltage. This transmembrane voltage ''is'' the membrane potential. As potassium continues to leave the cell, separating more charges, the membrane potential will continue to grow. The length of the arrows (green indicating concentration gradient, red indicating voltage), represents the magnitude of potassium ion movement due to each form of energy. The direction of the arrow indicates the direction in which that particular force is applied. Thus, the building membrane voltage is an increasing force that acts counter to the tendency for net movement of potassium ions down the potassium concentration gradient.
*In Panel 3, the membrane voltage has grown to the extent that its "strength" now matches the concentration gradients. Since these forces (which are applied to K+) are now the same strength and oriented in opposite directions, the system is now in equilibrium. Put another way, the tendency of potassium to leave the cell by running down its concentration gradient is now matched by the tendency of the membrane voltage to pull potassium ions back into the cell. K+ continues to move across the membrane, but the rate at which it enters and leaves the cell are the same, thus, there is no net potassium current. Because the K+ is at equilibrium, membrane potential is stable, or "resting" (EK).
The resting voltage is the result of several ion-translocating enzymes ( uniporters, cotransporter
Cotransporters are a subcategory of membrane transport proteins (transporters) that couple the favorable movement of one molecule with its concentration gradient and unfavorable movement of another molecule against its concentration gradient. They ...
s, and pumps) in the plasma membrane, steadily operating in parallel, whereby each ion-translocator has its characteristic electromotive force
In electromagnetism and electronics, electromotive force (also electromotance, abbreviated emf, denoted \mathcal) is an energy transfer to an electric circuit per unit of electric charge, measured in volts. Devices called electrical ''transducer ...
(= reversal potential = 'equilibrium voltage'), depending on the particular substrate concentrations inside and outside (internal ATP included in case of some pumps). H+ exporting ATPase
ATPases (, Adenosine 5'-TriPhosphatase, adenylpyrophosphatase, ATP monophosphatase, triphosphatase, ATP hydrolase, adenosine triphosphatase) are a class of enzymes that catalyze the decomposition of ATP into ADP and a free phosphate ion or ...
render the membrane voltage in plants and fungi much more negative than in the more extensively investigated animal cells, where the resting voltage is mainly determined by selective ion channels.
In most neurons the resting potential has a value of approximately −70 mV. The resting potential is mostly determined by the concentrations of the ions in the fluids on both sides of the cell membrane
The cell membrane (also known as the plasma membrane or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of a cell from the outside environment (the extr ...
and the ion transport protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
s that are in the cell membrane. How the concentrations of ions and the membrane transport proteins influence the value of the resting potential is outlined below.
The resting potential of a cell can be most thoroughly understood by thinking of it in terms of equilibrium potentials. In the example diagram here, the model cell was given only one permeant ion (potassium). In this case, the resting potential of this cell would be the same as the equilibrium potential for potassium.
However, a real cell is more complicated, having permeabilities to many ions, each of which contributes to the resting potential. To understand better, consider a cell with only two permeant ions, potassium, and sodium. Consider a case where these two ions have equal concentration gradients directed in opposite directions, and that the membrane permeabilities to both ions are equal. K+ leaving the cell will tend to drag the membrane potential toward ''E''K. Na+ entering the cell will tend to drag the membrane potential toward the reversal potential for sodium ''E''Na. Since the permeabilities to both ions were set to be equal, the membrane potential will, at the end of the Na+/K+ tug-of-war, end up halfway between ''E''Na and ''E''K. As ''E''Na and ''E''K were equal but of opposite signs, halfway in between is zero, meaning that the membrane will rest at 0 mV.Note that even though the membrane potential at 0 mV is stable, it is not an equilibrium condition because neither of the contributing ions is in equilibrium. Ions diffuse down their electrochemical gradients through ion channels, but the membrane potential is upheld by continual K+ influx and Na+ efflux via ion transporters. Such situation with similar permeabilities for counter-acting ions, like potassium and sodium in animal cells, can be extremely costly for the cell if these permeabilities are relatively large, as it takes a lot of ATP energy to pump the ions back. Because no real cell can afford such equal and large ionic permeabilities at rest, resting potential of animal cells is determined by predominant high permeability to potassium and adjusted to the required value by modulating sodium and chloride permeabilities and gradients. In a healthy animal cell Na+ permeability is about 5% of the K+ permeability or even less, whereas the respective reversal potentials are +60 mV for sodium (''E''Na)and −80 mV for potassium (''E''K). Thus the membrane potential will not be right at ''E''K, but rather depolarized from ''E''K by an amount of approximately 5% of the 140 mV difference between ''E''K and ''E''Na. Thus, the cell's resting potential will be about −73 mV. In a more formal notation, the membrane potential is the
weighted average
The weighted arithmetic mean is similar to an ordinary arithmetic mean (the most common type of average), except that instead of each of the data points contributing equally to the final average, some data points contribute more than others. The ...
of each contributing ion's equilibrium potential. The size of each weight is the relative conductance of each ion. In the normal case, where three ions contribute to the membrane potential:
:,
where
*''E''m is the membrane potential, measured in volts
*''E''X is the equilibrium potential for ion X, also in volts
*''g''X/''g''tot is the relative conductance of ion X, which is dimensionless
*''g''tot is the total conductance of all permeant ions in arbitrary units (e.g. siemens
Siemens AG ( ) is a German multinational technology conglomerate. It is focused on industrial automation, building automation, rail transport and health technology. Siemens is the largest engineering company in Europe, and holds the positi ...
for electrical conductance), in this case ''g''K+ + ''g''Na+ + ''g''Cl−
Membrane transport proteins
For determination of membrane potentials, the two most important types of membrane ion transport proteins areion channel
Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by Gating (electrophysiol ...
s and ion transporters. Ion channel proteins create paths across cell membranes through which ions can passively diffuse
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical p ...
without direct expenditure of metabolic energy. They have selectivity for certain ions, thus, there are potassium-, chloride-, and sodium-selective ion channels. Different cells and even different parts of one cell (dendrite
A dendrite (from Ancient Greek language, Greek δένδρον ''déndron'', "tree") or dendron is a branched cytoplasmic process that extends from a nerve cell that propagates the neurotransmission, electrochemical stimulation received from oth ...
s, cell bodies, nodes of Ranvier
Nodes of Ranvier ( ), also known as myelin-sheath gaps, occur along a myelinated axon where the axolemma is exposed to the extracellular space. Nodes of Ranvier are uninsulated axonal domains that are high in sodium and potassium ion channels ...
) will have different amounts of various ion transport proteins. Typically, the amount of certain potassium channels is most important for control of the resting potential (see below). Some ion pumps such as the Na+/K+-ATPase are electrogenic, that is, they produce charge imbalance across the cell membrane and can also contribute directly to the membrane potential. Most pumps use metabolic energy (ATP) to function.
Equilibrium potentials
For most animal cellspotassium
Potassium is a chemical element; it has Symbol (chemistry), symbol K (from Neo-Latin ) and atomic number19. It is a silvery white metal that is soft enough to easily cut with a knife. Potassium metal reacts rapidly with atmospheric oxygen to ...
ions (K+) are the most important for the resting potential.Aexample
of an
electrophysiological
Electrophysiology (from ee the Electron#Etymology, etymology of "electron" ; and ) is the branch of physiology that studies the electrical properties of biological cell (biology), cells and tissues. It involves measurements of voltage change ...
experiment to demonstrate the importance of K+ for the resting potential. The dependence of the resting potential on the extracellular concentration of K+ is shown in Figure 2.6 of ''Neuroscience'', 2nd edition, by Dale Purves, George J. Augustine, David Fitzpatrick, Lawrence C. Katz, Anthony-Samuel LaMantia, James O. McNamara, S. Mark Williams. Sunderland (MA): Sinauer Associates, Inc.; 2001. Due to the active transport
In cellular biology, active transport is the movement of molecules or ions across a cell membrane from a region of lower concentration to a region of higher concentration—against the concentration gradient. Active transport requires cellula ...
of potassium ions, the concentration of potassium is higher inside cells than outside. Most cells have potassium-selective ion channel proteins that remain open all the time. There will be net movement of positively charged potassium ions through these potassium channels with a resulting accumulation of excess negative charge inside of the cell. The outward movement of positively charged potassium ions is due to random molecular motion (diffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical p ...
) and continues until enough excess negative charge accumulates inside the cell to form a membrane potential which can balance the difference in concentration of potassium between inside and outside the cell. "Balance" means that the electrical force (potential
Potential generally refers to a currently unrealized ability. The term is used in a wide variety of fields, from physics to the social sciences to indicate things that are in a state where they are able to change in ways ranging from the simple r ...
) that results from the build-up of ionic charge, and which impedes outward diffusion, increases until it is equal in magnitude but opposite in direction to the tendency for outward diffusive movement of potassium. This balance point is an ''equilibrium potential In a biological membrane, the reversal potential is the membrane potential at which the direction of ionic current reverses. At the reversal potential, there is no net flow of ions from one side of the membrane to the other. For channels that are pe ...
'' as the net transmembrane flux (or current) of K+ is zero. A good approximation for the equilibrium potential of a given ion only needs the concentrations on either side of the membrane and the temperature. It can be calculated using the Nernst equation:
:
where
*''E''eq,K+ is the equilibrium potential for potassium, measured in volt
The volt (symbol: V) is the unit of electric potential, Voltage#Galvani potential vs. electrochemical potential, electric potential difference (voltage), and electromotive force in the International System of Units, International System of Uni ...
s
*''R'' is the universal gas constant
The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol or . It is the molar equivalent to the Boltzmann constant, expressed in units of energy per temperature increment p ...
, equal to 8.314 joule
The joule ( , or ; symbol: J) is the unit of energy in the International System of Units (SI). In terms of SI base units, one joule corresponds to one kilogram- metre squared per second squared One joule is equal to the amount of work d ...
s·K−1·mol−1
*''T'' is the absolute temperature
Thermodynamic temperature, also known as absolute temperature, is a physical quantity which measures temperature starting from absolute zero, the point at which particles have minimal thermal motion.
Thermodynamic temperature is typically expres ...
, measured in kelvin
The kelvin (symbol: K) is the base unit for temperature in the International System of Units (SI). The Kelvin scale is an absolute temperature scale that starts at the lowest possible temperature (absolute zero), taken to be 0 K. By de ...
s (= K = degrees Celsius + 273.15)
*''z'' is the number of elementary charge
The elementary charge, usually denoted by , is a fundamental physical constant, defined as the electric charge carried by a single proton (+1 ''e'') or, equivalently, the magnitude of the negative electric charge carried by a single electron, ...
s of the ion in question involved in the reaction
*''F'' is the Faraday constant
In physical chemistry, the Faraday constant (symbol , sometimes stylized as ℱ) is a physical constant defined as the quotient of the total electric charge () by the amount () of elementary charge carriers in any given sample of matter: it ...
, equal to 96,485 coulomb
The coulomb (symbol: C) is the unit of electric charge in the International System of Units (SI).
It is defined to be equal to the electric charge delivered by a 1 ampere current in 1 second, with the elementary charge ''e'' as a defining c ...
s·mol−1 or J·V−1·mol−1
* +sub>o is the extracellular concentration of potassium, measured in mol·m−3 or mmol·l−1
* +sub>i is likewise the intracellular concentration of potassium
Potassium equilibrium potentials of around −80 millivolts (inside negative) are common. Differences are observed in different species, different tissues within the same animal, and the same tissues under different environmental conditions. Applying the Nernst Equation above, one may account for these differences by changes in relative K+ concentration or differences in temperature.
For common usage the Nernst equation is often given in a simplified form by assuming typical human body temperature (37 °C), reducing the constants and switching to Log base 10. (The units used for concentration are unimportant as they will cancel out into a ratio). For Potassium at normal body temperature one may calculate the equilibrium potential in millivolts as:
:
Likewise the equilibrium potential for sodium (Na+) at normal human body temperature is calculated using the same simplified constant. You can calculate E assuming an outside concentration, +sub>o, of 10mM and an inside concentration, +sub>i, of 100mM. For chloride ions (Cl−) the sign of the constant must be reversed (−61.54 mV). If calculating the equilibrium potential for calcium (Ca2+) the 2+ charge halves the simplified constant to 30.77 mV. If working at room temperature, about 21 °C, the calculated constants are approximately 58 mV for K+ and Na+, −58 mV for Cl− and 29 mV for Ca2+. At physiological temperature, about 29.5 °C, and physiological concentrations (which vary for each ion), the calculated potentials are approximately 67 mV for Na+, −90 mV for K+, −86 mV for Cl− and 123 mV for Ca2+.
Resting potentials
The resting membrane potential is not an equilibrium potential as it relies on the constant expenditure of energy (for ionic pumps as mentioned above) for its maintenance. It is a dynamic diffusion potential that takes this mechanism into account—wholly unlike the pillows equilibrium potential, which is true no matter the nature of the system under consideration. The resting membrane potential is dominated by the ionic species in the system that has the greatest conductance across the membrane. For most cells this is potassium. As potassium is also the ion with the most negative equilibrium potential, usually the resting potential can be no more negative than the potassium equilibrium potential. The resting potential can be calculated with the Goldman-Hodgkin-Katz voltage equation using the concentrations of ions as for the equilibrium potential while also including the relative permeabilities of each ionic species. Under normal conditions, it is safe to assume that only potassium,sodium
Sodium is a chemical element; it has Symbol (chemistry), symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 element, group 1 of the peri ...
(Na+) and chloride
The term chloride refers to a compound or molecule that contains either a chlorine anion (), which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond (). The pr ...
(Cl−) ions play large roles for the resting potential:
:
This equation resembles the Nernst equation, but has a term for each permeant ion. Also, ''z'' has been inserted into the equation, causing the intracellular and extracellular concentrations of Cl− to be reversed relative to K+ and Na+, as chloride's negative charge is handled by inverting the fraction inside the logarithmic term. *''E''m is the membrane potential, measured in volts *''R'', ''T'', and ''F'' are as above *''P''s is the relative permeability of ion s * sub>Y is the concentration of ion s in compartment Y as above. Another way to view the membrane potential, considering instead the conductance of the ion channels rather than the permeability of the membrane, is using the Millman equation (also called the Chord Conductance Equation):
:
or reformulated
:
where ''g''tot is the combined conductance of all ionic species, again in arbitrary units. The latter equation portrays the resting membrane potential as a ''weighted average
The weighted arithmetic mean is similar to an ordinary arithmetic mean (the most common type of average), except that instead of each of the data points contributing equally to the final average, some data points contribute more than others. The ...
'' of the reversal potentials of the system, where the weights are the relative conductances of each ion species (''g''X/''g''tot). During the action potential, these weights change. If the conductances of Na+ and Cl− are zero, the membrane potential reduces to the Nernst potential for K+ (as ''g''K+ = ''g''tot). Normally, under resting conditions ''g''Na+ and ''g''Cl− are not zero, but they are much smaller than ''g''K+, which renders ''E''m close to ''E''eq,K+. Medical conditions such as hyperkalemia
Hyperkalemia is an elevated level of potassium (K+) in the blood. Normal potassium levels are between 3.5 and 5.0 mmol/L (3.5 and 5.0 mEq/L) with levels above 5.5mmol/L defined as hyperkalemia. Typically hyperkalemia does not cause symptoms. Oc ...
in which blood
Blood is a body fluid in the circulatory system of humans and other vertebrates that delivers necessary substances such as nutrients and oxygen to the cells, and transports metabolic waste products away from those same cells.
Blood is com ...
serum potassium (which governs +sub>o) is changed are very dangerous since they offset ''E''eq,K+, thus affecting ''E''m. This may cause arrhythmia
Arrhythmias, also known as cardiac arrhythmias, are irregularities in the cardiac cycle, heartbeat, including when it is too fast or too slow. Essentially, this is anything but normal sinus rhythm. A resting heart rate that is too fast – ab ...
s and cardiac arrest
Cardiac arrest (also known as sudden cardiac arrest CA is when the heart suddenly and unexpectedly stops beating. When the heart stops beating, blood cannot properly Circulatory system, circulate around the body and the blood flow to the ...
. The use of a bolus injection of potassium chloride in executions by lethal injection
Lethal injection is the practice of injecting one or more drugs into a person (typically a barbiturate, paralytic, and potassium) for the express purpose of causing death. The main application for this procedure is capital punishment, but t ...
stops the heart by shifting the resting potential to a more positive value, which depolarizes and contracts the cardiac cells permanently, not allowing the heart to repolarize and thus enter diastole
Diastole ( ) is the relaxed phase of the cardiac cycle when the chambers of the heart are refilling with blood. The contrasting phase is systole when the heart chambers are contracting. Atrial diastole is the relaxing of the atria, and ventricul ...
to be refilled with blood.
Although the GHK voltage equation and Millman's equation are related, they are not equivalent. The critical difference is that Millman's equation assumes the current-voltage relationship to be ohmic, whereas the GHK voltage equation takes into consideration the small, instantaneous rectifications predicted by the GHK flux equation caused by the concentration gradient of ions. Thus, a more accurate estimate of membrane potential can be calculated using the GHK equation than with Millman's equation.
Measuring resting potentials
In some cells, the membrane potential is always changing (such as cardiac pacemaker cells). For such cells there is never any "rest" and the "resting potential" is a theoretical concept. Other cells with little in the way of membrane transport functions that change with time have a resting membrane potential that can be measured by inserting an electrode into the cell.An illustrated example of measuring membrane potentials with electrodes is in Figure 2.1 of ''Neuroscience'' by Dale Purves, et al. (see reference #1, above). Transmembrane potentials can also be measured optically with dyes that change their optical properties according to the membrane potential.Summary of resting potential values in different types of cells
History
Resting currents in nerves were measured and described by Julius Bernstein in 1902 where he proposed a "Membrane Theory" that explained the resting potential of nerve and muscle as a diffusion potential.See also
*Action potential
An action potential (also known as a nerve impulse or "spike" when in a neuron) is a series of quick changes in voltage across a cell membrane. An action potential occurs when the membrane potential of a specific Cell (biology), cell rapidly ri ...
* Depolarization
In biology, depolarization or hypopolarization is a change within a cell (biology), cell, during which the cell undergoes a shift in electric charge distribution, resulting in less negative charge inside the cell compared to the outside. Depolar ...
* Hyperpolarization (biology)
Hyperpolarization is a change in a cell's membrane potential that makes it more negative. Cells typically have a negative resting potential, with neuronal action potentials depolarizing the membrane. When the resting membrane potential is made ...
* Membrane potential
Membrane potential (also transmembrane potential or membrane voltage) is the difference in electric potential between the interior and the exterior of a biological cell. It equals the interior potential minus the exterior potential. This is th ...
References
External links
Neuroscience
- online textbook by Purves, et al.
Basic Neurochemistry
Molecular, Cellular, and Medical Aspects by Siegel, et al. *
Bertil Hille
Bertil Hille (born October 10, 1940) is an Emeritus Professor, and the Wayne E. Crill Endowed Professor in the Department of Physiology and Biophysics at the University of Washington. He is particularly well known for his pioneering research on ce ...
''Ion channels of excitable membranes'', 3rd ed., Sinauer Associates, Sunderland, MA (2001).
*
Resting Membrane Potential
- Online lecture notes on the resting membrane potential
- Online interactive tutorial {{DEFAULTSORT:Resting Potential Neurophysiology Membrane biology Potentials