randomised controlled trial on:
[Wikipedia]
[Google]
[Amazon]
 A randomized controlled trial (or randomized control trial; RCT) is a form of scientific experiment used to control factors not under direct experimental control. Examples of RCTs are
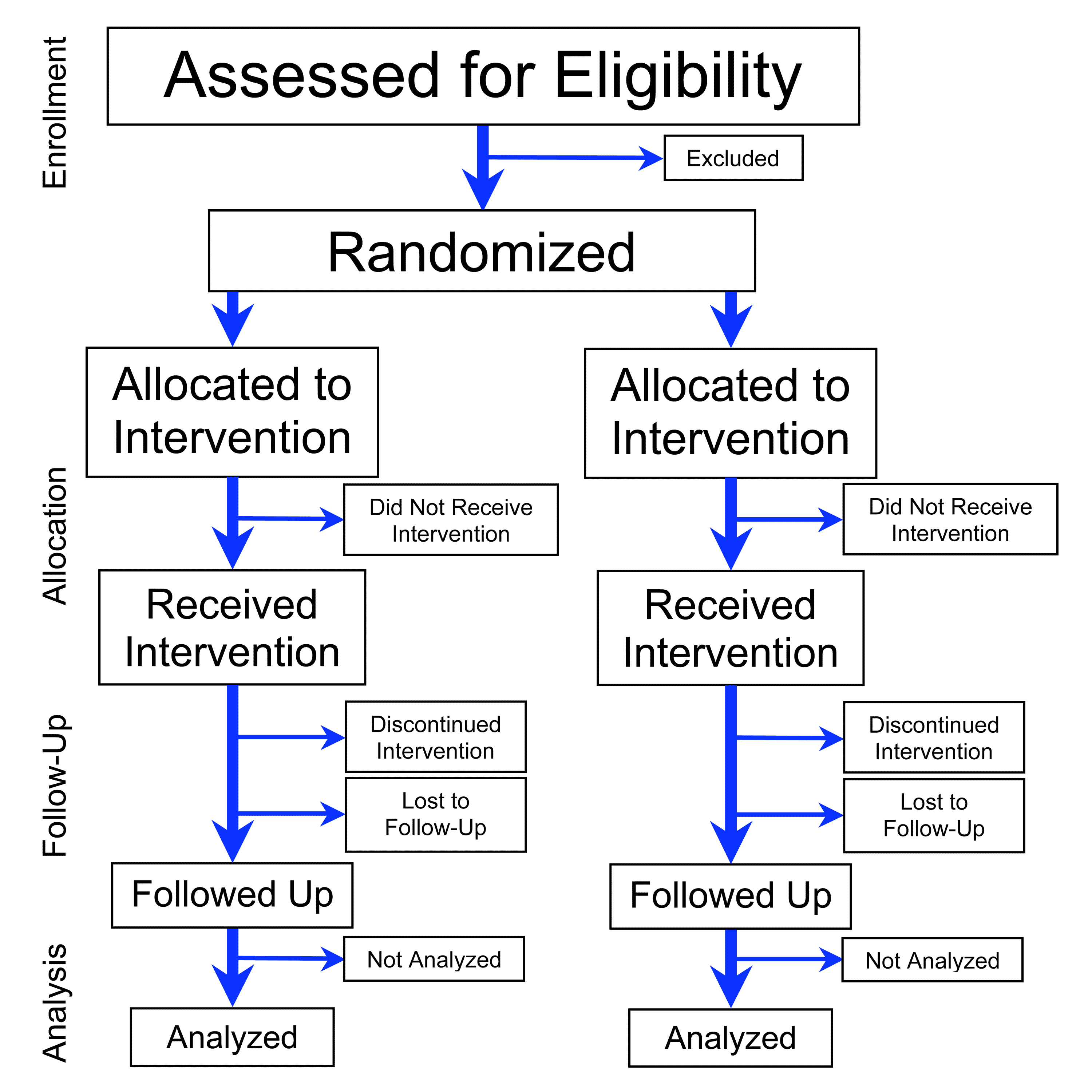
A randomized controlled trial (or randomized control trial; RCT) is a form of scientific experiment used to control factors not under direct experimental control. Examples of RCTs are
 A randomized controlled trial (or randomized control trial; RCT) is a form of scientific experiment used to control factors not under direct experimental control. Examples of RCTs are
A randomized controlled trial (or randomized control trial; RCT) is a form of scientific experiment used to control factors not under direct experimental control. Examples of RCTs are clinical trial
Clinical trials are prospective biomedical or behavioral research studies on human subject research, human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel v ...
s that compare the effects of drugs, surgical techniques, medical device
A medical device is any device intended to be used for medical purposes. Significant potential for hazards are inherent when using a device for medical purposes and thus medical devices must be proved safe and effective with reasonable assur ...
s, diagnostic procedures or other medical treatments.
Participants who enroll in RCTs differ from one another in known and unknown ways that can influence study outcomes, and yet cannot be directly controlled. By randomly allocating participants among compared treatments, an RCT enables ''statistical control'' over these influences. Provided it is designed well, conducted properly, and enrolls enough participants, an RCT may achieve sufficient control over these confounding factors to deliver a useful comparison of the treatments studied.
Definition and examples
An RCT inclinical research
Clinical research is a branch of healthcare science that determines the safety and effectiveness ( efficacy) of medications, devices, diagnostic products and treatment regimens intended for human use. These may be used for prevention, treat ...
typically compares a proposed new treatment against an existing standard of care; these are then termed the 'experimental' and 'control' treatments, respectively. When no such generally accepted treatment is available, a placebo
A placebo ( ) is a substance or treatment which is designed to have no therapeutic value. Common placebos include inert tablets (like sugar pills), inert injections (like Saline (medicine), sali