Radical Disproportionation on:
[Wikipedia]
[Google]
[Amazon]
Radical disproportionation encompasses a group of reactions in

\underset
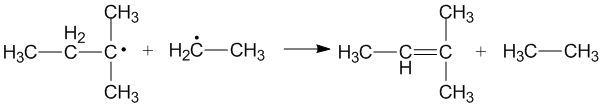

organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; ...
in which two radicals react to form two different non-radical products. Radicals in chemistry are defined as reactive atoms
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, an ...
or molecules
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioche ...
that contain an unpaired electron or electrons
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no ...
in an open shell. The unpaired electrons can cause radicals to be unstable and reactive. Reactions in radical chemistry can generate both radical and non-radical products
Product may refer to:
Business
* Product (business), an item that serves as a solution to a specific consumer problem.
* Product (project management), a deliverable or set of deliverables that contribute to a business solution
Mathematics
* Produ ...
. Radical disproportionation reactions can occur with many radicals in solution
Solution may refer to:
* Solution (chemistry), a mixture where one substance is dissolved in another
* Solution (equation), in mathematics
** Numerical solution, in numerical analysis, approximate solutions within specified error bounds
* Soluti ...
and in the gas phase
In the physical sciences, a phase is a region of space (a thermodynamic system), throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, magnetiza ...
. Due to the reactive nature of radical molecules, disproportionation
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation states. More generally, the term can b ...
proceeds rapidly and requires little to no activation energy
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules pe ...
.Thommarson, R. L. '' J. Phys. Chem.'', 1970, ''74'', 938-941. The most thoroughly studied radical disproportionation reactions have been conducted with alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloalk ...
radicals, but there are many organic molecules that can exhibit more complex, multi-step disproportionation reactions.
Mechanism of radical disproportionation
In radical disproportionation reactions one molecule acts as an acceptor while the other molecule acts as a donor.Benson, Sidney W. '' J. Phys. Chem.'', 1985, ''89'', 4366-4369. In the most common disproportionation reactions, a hydrogen atom is taken, or abstracted by the acceptor as the donor molecule undergoes anelimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 ...
to form a double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
.Kelley, Richard D., Klein, Ralph. '' J. Phys. Chem.'', 1974, ''78'', 1586-1595. Other atoms such as halogens may also be abstracted during a disproportionation reaction. Abstraction occurs as a head to tail reaction with the atom that is being abstracted facing the radical atom on the other molecule.

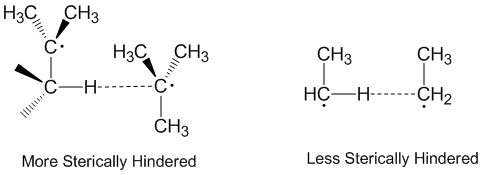
Disproportionation and steric effects
Radical disproportionation is often thought of as occurring in a linear fashion with the donor radical, the acceptor radical, and the atom being accepted all along the same axis. In fact, most disproportionation reactions do not require linear orientations in space. Molecules that are more sterically hindered require arrangements that are more linear, and thus react more slowly.Steric effects
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions ...
play a significant role in disproportionation with ethyl
Ethyl may refer to:
Arts and entertainment
* Cold Ethyl, a Swedish rock band
*Ethyl Sinclair, a character in the ''Dinosaurs'' television show
Science and technology
* Ethyl group, an organic chemistry moiety
* Ethyl alcohol (or ethanol)
* E ...
radicals acting as more effective acceptors than tert-butyl
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula , derived from either of the two isomers (''n''-butane and isobutane) of butane.
The isomer ''n''-butane can connect in two ways, givi ...
radicals. Tert-butyl radicals have many hydrogens on adjacent carbons to donate and steric effects often prevent tert-butyl radicals from getting close to abstracting hydrogens.

Alkyl radical disproportionation
Alkyl radical disproportionation has been studied extensively in scientific literature.Gibian, Morton J. and Robert C. Corley. ''Chem. Rev.
''Chemical Reviews'' is peer-reviewed scientific journal published twice per month by the American Chemical Society. It publishes review articles on all aspects of chemistry. It was established in 1924 by William Albert Noyes (University of Illinoi ...
'', 1973, ''73'', 441-464. During alkyl radical disproportionation, an alkane and an alkene are the end products and the bond order
In chemistry, bond order, as introduced by Linus Pauling, is defined as the difference between the number of bonds and anti-bonds.
The bond order itself is the number of electron pairs (covalent bonds) between two atoms. For example, in diat ...
of the products increases by one over the reactants. Thus the reaction is exothermic
In thermodynamics, an exothermic process () is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity (e ...
(ΔH = 50 – 95 kcal/mol) and proceeds rapidly.
:Cross disproportionation of alkyl radicals
Cross disproportionation occurs when two different alkyl radicals disproportionate to form two new products. Different products can be formed depending on which alkyl radical acts as a donor and which acts as an acceptor. The efficiency of primary and secondary alkyl radicals as donors depends on the steric effects and configuration of the radical acceptors.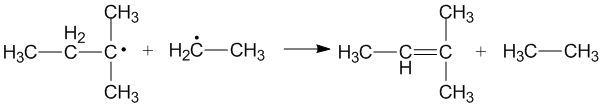
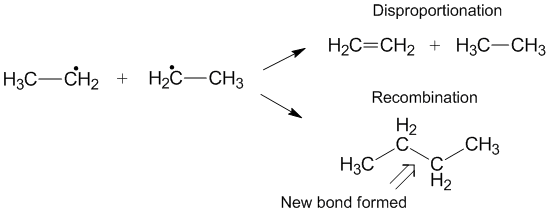
Competition with recombination
Another reaction that can sometimes occur instead of disproportionation is recombination. During recombination, two radicals form one new non-radical product and one new bond. Similar to disproportionation, the recombination reaction is exothermic and requires little to no activation energy. The ratio of the rates of disproportionation to recombination is referred to as kD/kC and often favors recombination compared with disproportionation for alkyl radicals. As the number of transferable hydrogens increase, therate constant In chemical kinetics a reaction rate constant or reaction rate coefficient, ''k'', quantifies the rate and direction of a chemical reaction.
For a reaction between reactants A and B to form product C
the reaction rate is often found to have the f ...
for disproportionation increases relative to the rate constant for recombination.

Kinetic isotope effect on disproportionation and recombination
When the hydrogen atoms in an alkyl radical are displaced withdeuterium
Deuterium (or hydrogen-2, symbol or deuterium, also known as heavy hydrogen) is one of two Stable isotope ratio, stable isotopes of hydrogen (the other being Hydrogen atom, protium, or hydrogen-1). The atomic nucleus, nucleus of a deuterium ato ...
, disproportionation proceeds at a slightly slower rate whereas the rate of recombination remains the same. Thus disproportionation is weakly affected by the kinetic isotope effect
In physical organic chemistry, a kinetic isotope effect (KIE) is the change in the reaction rate of a chemical reaction when one of the atoms in the reactants is replaced by one of its isotopes. Formally, it is the ratio of rate constants for th ...
with kH/kD = 1.20 ± 0.15 for ethylene. Hydrogens and deuterons are not involved in recombination reactions. However, deuteron abstraction during disproportionation occurs more slowly than hydrogen abstraction due to the increased mass and reduced vibrational energy of deuterium, although the experimentally observed kH/kD is close to one.
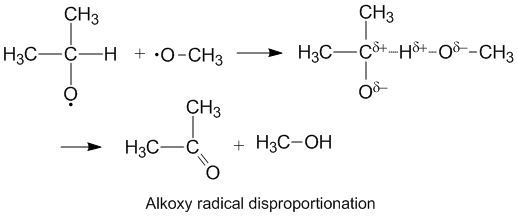
Polar effects and alkoxy radical disproportionation
Alkoxy
In chemistry, the alkoxy group is an alkyl group which is singularly bonded to oxygen; thus . The range of alkoxy groups is vast, the simplest being methoxy (). An ethoxy group () is found in the organic compound ethyl phenyl ether (, also ...
radicals which contain unpaired electrons on an oxygen atom display a higher kD/kC compared to alkyl radicals. The oxygen has a partial negative charge
Charge or charged may refer to:
Arts, entertainment, and media Films
* '' Charge, Zero Emissions/Maximum Speed'', a 2011 documentary
Music
* ''Charge'' (David Ford album)
* ''Charge'' (Machel Montano album)
* ''Charge!!'', an album by The Aqu ...
which removes electron density from the donor carbon atom thereby facilitating hydrogen abstraction. The rate of disproportionation is also aided by the more electronegative
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
oxygen on the acceptor molecule.

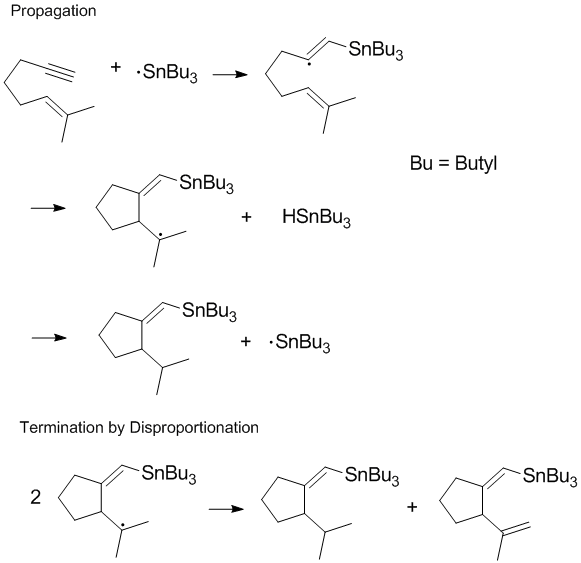
Termination of chain processes
Many radical processes involvechain reaction
A chain reaction is a sequence of reactions where a reactive product or by-product causes additional reactions to take place. In a chain reaction, positive feedback leads to a self-amplifying chain of events.
Chain reactions are one way that syst ...
s or chain propagation with disproportionation and recombination occurring in the terminal step of the reaction.Matyjaszewski, Krysztof, Xia, Jianhui. ''Chem. Rev.
''Chemical Reviews'' is peer-reviewed scientific journal published twice per month by the American Chemical Society. It publishes review articles on all aspects of chemistry. It was established in 1924 by William Albert Noyes (University of Illinoi ...
'', 2001, ''101'', 2921-2990. Terminating chain propagation is often most significant during polymerization as the desired chain propagation cannot take place if disproportionation and recombination reactions readily occur. Controlling termination products and regulating disproportionation and recombination reactions in the terminal step are important considerations in radical chemistry and polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer, monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are ...
. In some reactions (such as the one shown below) one or both of the termination pathways can be hindered by steric or solvent effects
In chemistry, solvent effects are the influence of a solvent on chemical reactivity or molecular associations. Solvents can have an effect on solubility, stability and reaction rates and choosing the appropriate solvent allows for thermodynamic a ...
.

Reducing disproportionation in living free radical polymerization
Many polymer chemists are concerned with limiting the rate of disproportionation during polymerization. Although disproportionation results in formation of one new double bond which may react with the polymer chain, a saturatedhydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ex ...
is also formed, and thus the chain reaction does not readily proceed. During living free radical polymerization
Living free radical polymerization is a type of living polymerization where the active polymer chain end is a free radical. Several methods exist. IUPAC recommends to use the term "reversible-deactivation radical polymerization" instead of "livi ...
, termination pathways for a growing polymer chain are removed. This can be achieved through several methods, one of which is reversible termination with stable radicals. Nitroxide
Aminoxyl denotes a radical functional group with general structure R2N–O•. It is commonly known as a nitroxyl radical or a nitroxide, however IUPAC discourages the use of these terms, as they erroneously suggest the presence of a nitro group. ...
radicals and other stable radicals reduce recombination and disproportionation rates and control the concentration of polymeric radicals.Kruse, Todd M., Souleimonova, Razima, Cho, Andrew, Gray, Maisha K., Torkelson, John M., Broadbelt, Linda J. ''Macromolecules
A macromolecule is a very large molecule important to biophysical processes, such as a protein or nucleic acid. It is composed of thousands of covalently bonded atoms. Many macromolecules are polymers of smaller molecules called monomers. The ...
'', 2003, ''36'', 7812-7823.

References
{{Reflist Organic chemistry