Prilezhaev reaction on:
[Wikipedia]
[Google]
[Amazon]
The Prilezhaev reaction, also known as the Prileschajew reaction or Prilezhaev epoxidation, is the  The oxygen atom that adds across the double bond of the alkene is taken from the peroxy acid, generating a molecule of the corresponding
The oxygen atom that adds across the double bond of the alkene is taken from the peroxy acid, generating a molecule of the corresponding
 The reaction proceeds through what is commonly known as the "butterfly mechanism", first proposed by Bartlett, wherein the peracid is intramolecularly hydrogen-bonded at the transition state. Although there are frontier orbital interactions in both directions, the peracid is generally viewed as the
The reaction proceeds through what is commonly known as the "butterfly mechanism", first proposed by Bartlett, wherein the peracid is intramolecularly hydrogen-bonded at the transition state. Although there are frontier orbital interactions in both directions, the peracid is generally viewed as the  There is a very large dependence of the reaction rate on the choice of solvent.
There is a very large dependence of the reaction rate on the choice of solvent.
chemical reaction
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking ...
of an alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic ...
with a peroxy acid
A peroxy acid (often spelled as one word, peroxyacid, and sometimes called peracid) is an acid which contains an acidic –OOH group. The two main classes are those derived from conventional mineral acids, especially sulfuric acid, and the perox ...
to form epoxide
In organic chemistry, an epoxide is a cyclic ether () with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale ...
s. It is named after Nikolai Prilezhaev, who first reported this reaction in 1909. A widely used peroxy acid
A peroxy acid (often spelled as one word, peroxyacid, and sometimes called peracid) is an acid which contains an acidic –OOH group. The two main classes are those derived from conventional mineral acids, especially sulfuric acid, and the perox ...
for this reaction is ''meta''-chloroperoxybenzoic acid (''m''-CPBA), due to its stability and good solubility in most organic solvents. The reaction is performed in inert solvents (C6H14, C6H6, CH2Cl2, CHCl3, CCl4) between -10 and 60 °C with the yield of 60-80%.
An illustrative example is the epoxidation of ''trans''-2-butene with ''m''-CPBA to give ''trans''-2,3-epoxybutane:
 The oxygen atom that adds across the double bond of the alkene is taken from the peroxy acid, generating a molecule of the corresponding
The oxygen atom that adds across the double bond of the alkene is taken from the peroxy acid, generating a molecule of the corresponding carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxyli ...
as a byproduct. The reaction is highly stereospecific in the sense that the double bond stereochemistry is generally transferred to the relative configuration of the epoxide with essentially perfect fidelity, so that a ''trans''-olefin leads to the stereoselective formation of the ''trans''-2,3-substituted epoxide only, as illustrated by the example above, while a ''cis''-olefin would only give the ''cis-''epoxide. This stereochemical outcome is a consequence of the accepted mechanism, discussed below.
Reaction mechanism
 The reaction proceeds through what is commonly known as the "butterfly mechanism", first proposed by Bartlett, wherein the peracid is intramolecularly hydrogen-bonded at the transition state. Although there are frontier orbital interactions in both directions, the peracid is generally viewed as the
The reaction proceeds through what is commonly known as the "butterfly mechanism", first proposed by Bartlett, wherein the peracid is intramolecularly hydrogen-bonded at the transition state. Although there are frontier orbital interactions in both directions, the peracid is generally viewed as the electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carrie ...
and the alkene as the nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they ar ...
. In support of this notion, more electron-rich alkenes undergo epoxidation at a faster rate. For example, the relative rates of epoxidation increase upon methyl substitution of the alkene (the methyl groups increase the electron density of the double bond by hyperconjugation): ethylene (1, no methyl groups), propene (24, one methyl group), ''cis''-2-butene (500, two methyl groups), 2-methyl-2-butene (6500, three methyl groups), 2,3-dimethyl-2-butene (>6500, four methyl groups).
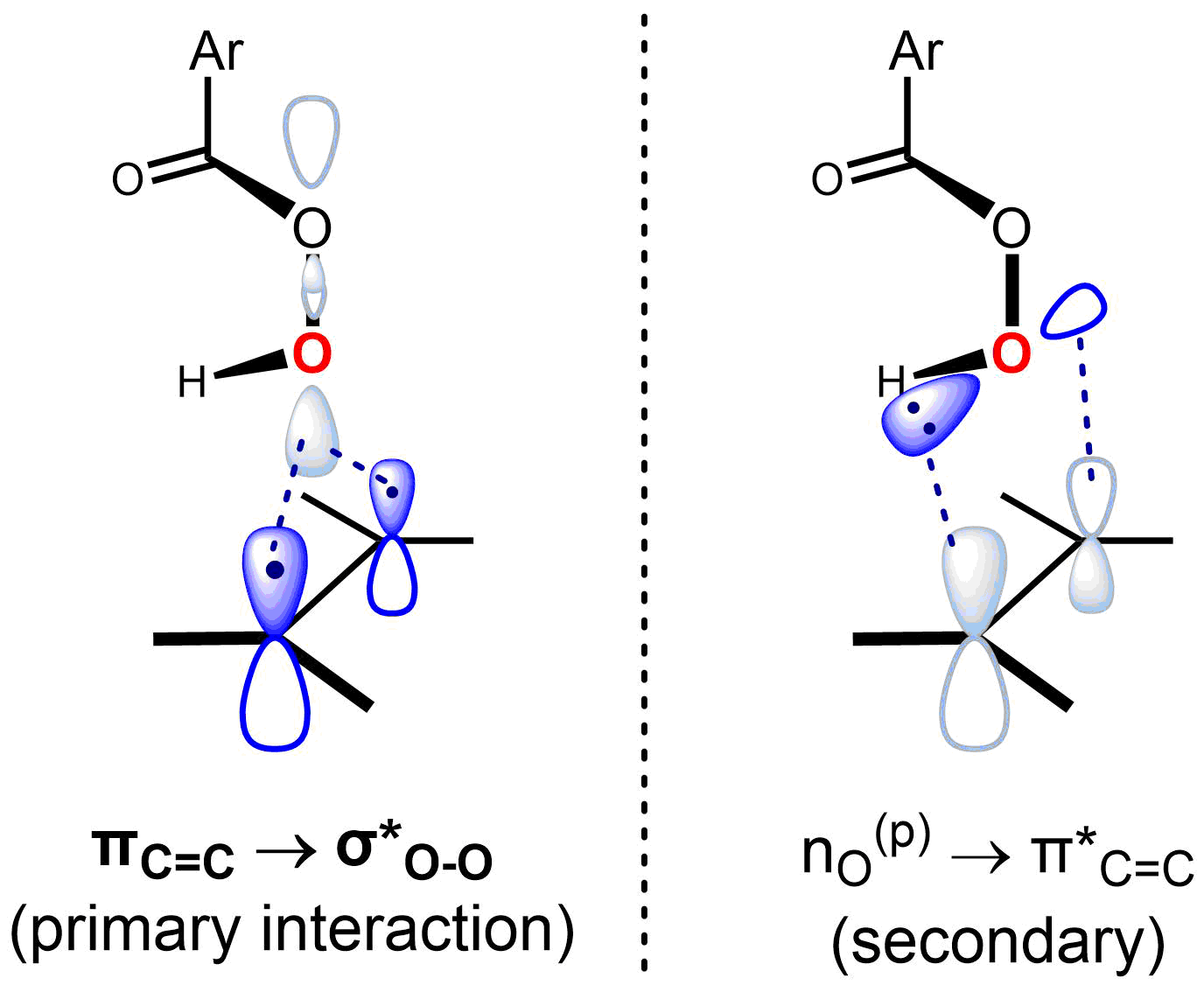
The reaction is believed to be concerted, with a transition state that is synchronous or nearly so. The "butterfly mechanism" takes place via a transition state geometry in which the plane of the peracid bisects that of the alkene, with the O–O bond aligned perpendicular to it. This conformation allows the key frontier orbital interactions to occur. The primary interaction of the occupied πC=C orbital (HOMO) and the low-lying unoccupied σ*O-O orbital (LUMO). This interaction accounts for the observed overall nucleophilic character and electrophilic character of the alkene and peracid, respectively. There is also a secondary interaction between a lone pair orbital perpendicular to the plane of the peracid, nO(p) (HOMO) and the unoccupied π*C=C orbital (LUMO). Using the approach of Anslyn and Dougherty (2006, p. 556), the mechanism can be represented as follows:
 There is a very large dependence of the reaction rate on the choice of solvent.
There is a very large dependence of the reaction rate on the choice of solvent.
References
{{Reflist Epoxidation reactions Oxygen heterocycle forming reactions Heterocycle forming reactions Name reactions