Polymer backbone on:
[Wikipedia]
[Google]
[Amazon]
In
 Common synthetic polymers have main chains composed of carbon, i.e. C-C-C-C.... Examples include
Common synthetic polymers have main chains composed of carbon, i.e. C-C-C-C.... Examples include

 Deoxyribonucleic acid (DNA) and
Deoxyribonucleic acid (DNA) and
polymer science
Polymer science or macromolecular science is a subfield of materials science concerned with polymers, primarily synthetic polymers such as plastics and elastomers. The field of polymer science includes researchers in multiple disciplines includ ...
, the polymer chain or simply backbone of a polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
is the main chain of a polymer. Polymers are often classified according to the elements in the main chains. The character of the backbone, i.e. its flexibility, determines the properties of the polymer (such as the glass transition
The glass–liquid transition, or glass transition, is the gradual and reversible transition in amorphous materials (or in amorphous regions within semicrystalline materials) from a hard and relatively brittle "glassy" state into a viscous or rubb ...
temperature). For example, in polysiloxanes (silicone), the backbone chain is very flexible, which results in a very low glass transition
The glass–liquid transition, or glass transition, is the gradual and reversible transition in amorphous materials (or in amorphous regions within semicrystalline materials) from a hard and relatively brittle "glassy" state into a viscous or rubb ...
temperature of . The polymers with rigid backbones are prone to crystallization
Crystallization is the process by which solid forms, where the atoms or molecules are highly organized into a structure known as a crystal. Some ways by which crystals form are precipitating from a solution, freezing, or more rarely deposi ...
(e.g. polythiophenes) in thin film
A thin film is a layer of material ranging from fractions of a nanometer (monolayer) to several micrometers in thickness. The controlled synthesis of materials as thin films (a process referred to as deposition) is a fundamental step in many ap ...
s and in solution
Solution may refer to:
* Solution (chemistry), a mixture where one substance is dissolved in another
* Solution (equation), in mathematics
** Numerical solution, in numerical analysis, approximate solutions within specified error bounds
* Soluti ...
. Crystallization in its turn affects the optical properties of the polymers, its optical band gap
In solid-state physics, a band gap, also called an energy gap, is an energy range in a solid where no electronic states can exist. In graphs of the electronic band structure of solids, the band gap generally refers to the energy difference (in ...
and electronic levels.
Organic polymers
:polyolefin
A polyolefin is a type of polymer with the general formula (CH2CHR)n where R is an alkyl group. They are usually derived from a small set of simple olefins (alkenes). Dominant in a commercial sense are polyethylene and polypropylene. More speciali ...
s such as polyethylene
Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging ( plastic bags, plastic films, geomembranes and containers including bo ...
((CH2CH2)n) and many substituted derivative ((CH2CH(R))n) such as polystyrene
Polystyrene (PS) is a synthetic polymer made from monomers of the aromatic hydrocarbon styrene. Polystyrene can be solid or foamed. General-purpose polystyrene is clear, hard, and brittle. It is an inexpensive resin per unit weight. It is a ...
(R = C6H5), polypropylene
Polypropylene (PP), also known as polypropene, is a thermoplastic polymer used in a wide variety of applications. It is produced via chain-growth polymerization from the monomer propylene.
Polypropylene
belongs to the group of polyolefins and ...
(R = CH3), and acrylate
Acrylates (IUPAC: prop-2-enoates) are the salts, esters, and conjugate bases of acrylic acid. The acrylate ion is the anion C H2=CHC OO−. Often, acrylate refers to esters of acrylic acid, the most common member being methyl acrylate. These acry ...
s (R = CO2R').
Other major classes of organic polymers are polyester
Polyester is a category of polymers that contain the ester functional group in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include natural ...
s and polyamide
A polyamide is a polymer with repeating units linked by amide bonds.
Polyamides occur both naturally and artificially. Examples of naturally occurring polyamides are proteins, such as wool and silk. Artificially made polyamides can be made through ...
s. They have respectively -C(O)-O- and -C(O)-NH- groups in their backbones in addition to chains of carbon. Major commercial products are polyethyleneterephthalate
Polyethylene terephthalate (or poly(ethylene terephthalate), PET, PETE, or the obsolete PETP or PET-P), is the most common thermoplastic polymer resin of the polyester family and is used in fibres for clothing, containers for liquids and foods, ...
("PET"), ((C6H4CO2C2H4OC(O))n) and nylon-6
Nylon 6 or polycaprolactam is a polymer, in particular semicrystalline polyamide. Unlike most other nylons, nylon 6 is not a condensation polymer, but instead is formed by ring-opening polymerization; this makes it a special case in the comparis ...
((NH(CH2)5C(O))n).
Inorganic polymers

Siloxane
A siloxane is a functional group in organosilicon chemistry with the Si−O−Si linkage. The parent siloxanes include the oligomeric and polymeric hydrides with the formulae H(OSiH2)''n''OH and (OSiH2)n. Siloxanes also include branched compoun ...
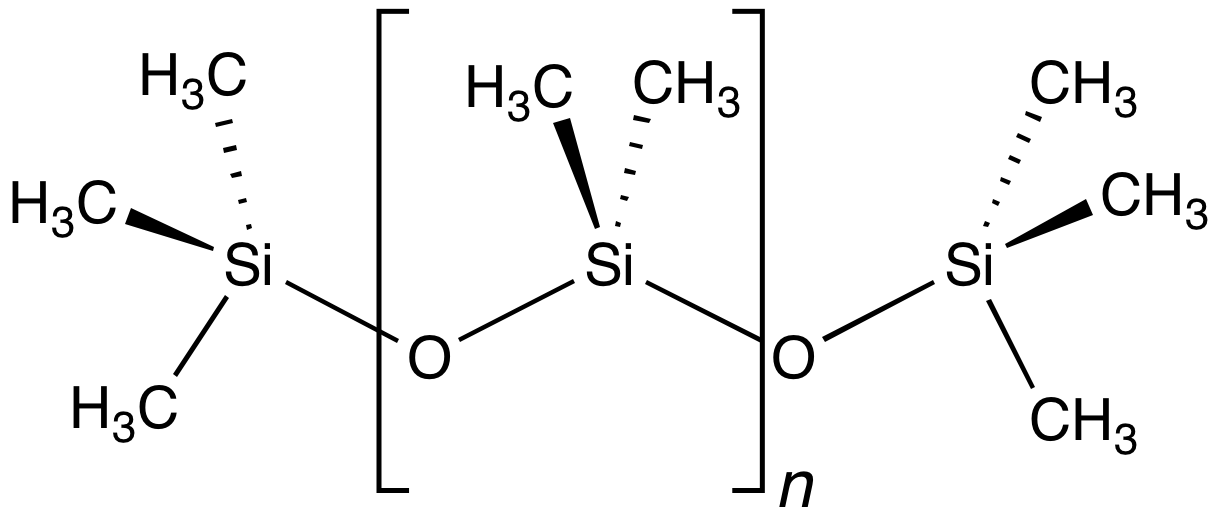
s are a premier example of an inorganic polymer, even though they have extensive organic substituents. Their backbond is composed of alternating silicon and oxygen atoms, i.e. Si-O-Si-O... The silicon atoms bear two substituents, usually methyl
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many ...
as in the case of polydimethylsiloxane
Polydimethylsiloxane (PDMS), also known as dimethylpolysiloxane or dimethicone, belongs to a group of polymeric organosilicon compounds that are commonly referred to as silicones. PDMS is the most widely used silicon-based organic polymer, as its ...
. Some uncommon but illustrative inorganic polymers include polythiazyl
Polythiazyl (polymeric sulfur nitride), , is an electrically conductive, gold- or bronze-colored polymer with metallic luster. It was the first conductive inorganic polymer discovered and was also found to be a superconductor at very low tempera ...
((SN)x) with alternating S and N atoms, and polyphosphates ((PO3−)n).
Biopolymers
Major families of biopolymers arepolysaccharide
Polysaccharides (), or polycarbohydrates, are the most abundant carbohydrates found in food. They are long chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with wa ...
s (carbohydrates), peptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.
A ...
s, and polynucleotide
A polynucleotide molecule is a biopolymer composed of 13 or more nucleotide
Nucleotides are organic molecules consisting of a nucleoside and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (D ...
s. Many variants of each are known.V
Proteins and peptides
Proteins are characterized by amide linkages (-N(H)-C(O)-) formed by the condensation ofamino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha am ...
s. The sequence of the amino acids in the polypeptide backbone is known as the primary structure
Protein primary structure is the linear sequence of amino acids in a peptide or protein. By convention, the primary structure of a protein is reported starting from the amino-terminal (N) end to the carboxyl-terminal (C) end. Protein biosynthes ...
of the protein. Like almost all polymers, protein fold and twist, forming into the secondary structure
Protein secondary structure is the three dimensional conformational isomerism, form of ''local segments'' of proteins. The two most common Protein structure#Secondary structure, secondary structural elements are alpha helix, alpha helices and beta ...
, which is rigidified by hydrogen bonding
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a l ...
between the carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a ...
oxygens and amide hydrogens in the backbone, i.e. C=O---HN. Further interactions between residues of the individual amino acids form the protein's tertiary structure
Protein tertiary structure is the three dimensional shape of a protein. The tertiary structure will have a single polypeptide chain "backbone" with one or more protein secondary structures, the protein domains. Amino acid side chains may int ...
. For this reason, the primary structure of the amino acids in the polypeptide backbone is the map of the final structure of a protein, and it therefore indicates its biological function. Spatial positions of backbone atoms can be reconstructed from the positions of alpha carbons using computational tools for the backbone reconstruction.
Carbohydrates
Carbohydrates arise by condensation ofmonosaccharide
Monosaccharides (from Greek ''monos'': single, '' sacchar'': sugar), also called simple sugars, are the simplest forms of sugar and the most basic units (monomers) from which all carbohydrates are built.
They are usually colorless, water-solub ...
s such as glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using ...
. The polymers can be classified into oligosaccharide
An oligosaccharide (/ˌɑlɪgoʊˈsækəˌɹaɪd/; from the Greek ὀλίγος ''olígos'', "a few", and σάκχαρ ''sácchar'', "sugar") is a saccharide polymer containing a small number (typically two to ten) of monosaccharides (simple sugar ...
s (up to 10 residues) and polysaccharide
Polysaccharides (), or polycarbohydrates, are the most abundant carbohydrates found in food. They are long chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with wa ...
s (up to about 50,000 residues). The backbone chain is characterized by an ether bond between individual monosaccharides. This bond is called the glycosidic linkage
A glycosidic bond or glycosidic linkage is a type of covalent bond that joins a carbohydrate (sugar) molecule to another group, which may or may not be another carbohydrate.
A glycosidic bond is formed between the hemiacetal or hemiketal grou ...
. These backbone chains can be unbranched (containing one linear chain) or branched (containing multiple chains). The glycosidic linkages are designated as ''alpha'' or ''beta'' depending on the relative stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereois ...
of the anomer
In carbohydrate chemistry, a pair of anomers () is a pair of near-identical stereoisomers that differ at only the anomeric carbon, the carbon that bears the aldehyde or ketone functional group in the sugar's open-chain form. However, in order fo ...
ic (or most oxidized
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
) carbon. In a Fischer Projection
In chemistry, the Fischer projection, devised by Emil Fischer in 1891, is a two-dimensional representation of a three-dimensional organic molecule by projection. Fischer projections were originally proposed for the depiction of carbohydrates an ...
, if the glycosidic linkage is on the same side or face as carbon 6 of a common biological saccharide, the carbohydrate is designated as ''beta'' and if the linkage is on the opposite side it is designated as ''alpha''. In a traditional " chair structure" projection, if the linkage is on the same plane (equatorial or axial) as carbon 6 it is designated as ''beta'' and on the opposite plane it is designated as ''alpha''. This is exemplified in sucrose
Sucrose, a disaccharide, is a sugar composed of glucose and fructose subunits. It is produced naturally in plants and is the main constituent of white sugar. It has the molecular formula .
For human consumption, sucrose is extracted and refined ...
(table sugar) which contains a linkage that is ''alpha'' to glucose and ''beta'' to fructose
Fructose, or fruit sugar, is a Ketose, ketonic monosaccharide, simple sugar found in many plants, where it is often bonded to glucose to form the disaccharide sucrose. It is one of the three dietary monosaccharides, along with glucose and galacto ...
. Generally, carbohydrates which our bodies break down are ''alpha''-linked (example: glycogen) and those which have structural function are ''beta''-linked (example: cellulose
Cellulose is an organic compound with the formula , a polysaccharide consisting of a linear chain of several hundred to many thousands of β(1→4) linked D-glucose units. Cellulose is an important structural component of the primary cell wall ...
).
Nucleic Acids
ribonucleic acid
Ribonucleic acid (RNA) is a polymeric molecule essential in various biological roles in coding, decoding, regulation and expression of genes. RNA and deoxyribonucleic acid ( DNA) are nucleic acids. Along with lipids, proteins, and carbohydra ...
(RNA) are the main examples of polynucleotide
A polynucleotide molecule is a biopolymer composed of 13 or more nucleotide
Nucleotides are organic molecules consisting of a nucleoside and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (D ...
s. They arise by condensation of nucleotides. Their backbones form by the condensation of a hydroxy group on a ribose
Ribose is a simple sugar and carbohydrate with molecular formula C5H10O5 and the linear-form composition H−(C=O)−(CHOH)4−H. The naturally-occurring form, , is a component of the ribonucleotides from which RNA is built, and so this compo ...
with the phosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid .
The phosphate or orthophosphate ion is derived from phospho ...
group on another ribose. This linkage is called a phosphodiester bond
In chemistry, a phosphodiester bond occurs when exactly two of the hydroxyl groups () in phosphoric acid react with hydroxyl groups on other molecules to form two ester bonds. The "bond" involves this linkage . Discussion of phosphodiesters is ...
. The condensation is catalyzed by enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
s called polymerase
A polymerase is an enzyme ( EC 2.7.7.6/7/19/48/49) that synthesizes long chains of polymers or nucleic acids. DNA polymerase and RNA polymerase are used to assemble DNA and RNA molecules, respectively, by copying a DNA template strand using base- ...
s. DNA and RNA can be millions of nucleotides long thus allowing for the genetic diversity
Genetic diversity is the total number of genetic characteristics in the genetic makeup of a species, it ranges widely from the number of species to differences within species and can be attributed to the span of survival for a species. It is dis ...
of life. The bases project from the pentose-phosphate polymer backbone and are hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
ed in pairs to their complementary
A complement is something that completes something else.
Complement may refer specifically to:
The arts
* Complement (music), an interval that, when added to another, spans an octave
** Aggregate complementation, the separation of pitch-class ...
partners (A with T and G with C). This creates a double helix
A double is a look-alike or doppelgänger; one person or being that resembles another.
Double, The Double or Dubble may also refer to:
Film and television
* Double (filmmaking), someone who substitutes for the credited actor of a character
* ...
with pentose phosphate backbones on either side, thus forming a secondary structure
Protein secondary structure is the three dimensional conformational isomerism, form of ''local segments'' of proteins. The two most common Protein structure#Secondary structure, secondary structural elements are alpha helix, alpha helices and beta ...
.
References
See also
*Pendant group
In IUPAC nomenclature of chemistry, a pendant group (sometimes spelled pendent) or side group is a group of atoms attached to a backbone chain of a long molecule, usually a polymer. Pendant groups are different from pendant chains, as they are n ...
* Peptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.
A ...
{{DEFAULTSORT:Backbone Chain
Organic chemistry