Pyridine Complexes on:
[Wikipedia]
[Google]
[Amazon]
Pyridine is a

 The molecular
The molecular
 Pyridine has a conjugated system of six π electrons that are delocalized over the ring. The molecule is planar and, thus, follows the Hückel criteria for aromatic systems. In contrast to benzene, the
Pyridine has a conjugated system of six π electrons that are delocalized over the ring. The molecule is planar and, thus, follows the Hückel criteria for aromatic systems. In contrast to benzene, the
 Impure pyridine was undoubtedly prepared by early
Impure pyridine was undoubtedly prepared by early  The contemporary methods of pyridine production had a low yield, and the increasing demand for the new compound urged to search for more efficient routes. A breakthrough came in 1924 when the Russian chemist
The contemporary methods of pyridine production had a low yield, and the increasing demand for the new compound urged to search for more efficient routes. A breakthrough came in 1924 when the Russian chemist
File:4-Bromopyridine.svg, 4-bromopyridine
File:2,2'-Bipyridine.svg, 2,2'-


 The trimerization of a part of a nitrile molecule and two parts of
The trimerization of a part of a nitrile molecule and two parts of
 The Ciamician–Dennstedt rearrangement entails the ring-expansion of pyrrole with dichlorocarbene to 3-Chloropyridine, 3-chloropyridine.
The Ciamician–Dennstedt rearrangement entails the ring-expansion of pyrrole with dichlorocarbene to 3-Chloropyridine, 3-chloropyridine.
 In the Gattermann–Skita synthesis, a Malonic ester synthesis, malonate ester salt reacts with dichloromethylamine.
In the Gattermann–Skita synthesis, a Malonic ester synthesis, malonate ester salt reacts with dichloromethylamine.
 Other methods include the Boger pyridine synthesis and Diels–Alder reaction of an alkene and an oxazole.
Other methods include the Boger pyridine synthesis and Diels–Alder reaction of an alkene and an oxazole.


 Direct nitration of pyridine is sluggish. Pyridine derivatives wherein the nitrogen atom is screened sterically and/or electronically can be obtained by nitration with nitronium tetrafluoroborate (NO2BF4). In this way, 3-nitropyridine can be obtained via the synthesis of 2,6-dibromopyridine followed by nitration and debromination.
Sulfonation of pyridine is even more difficult than nitration. However, pyridine-3-sulfonic acid can be obtained. Reaction with the SO3 group also facilitates addition of sulfur to the nitrogen atom, especially in the presence of a mercury(II) sulfate catalyst.
In contrast to the sluggish nitrations and sulfonations, the bromination and chlorination reaction, chlorination of pyridine proceed well.
Direct nitration of pyridine is sluggish. Pyridine derivatives wherein the nitrogen atom is screened sterically and/or electronically can be obtained by nitration with nitronium tetrafluoroborate (NO2BF4). In this way, 3-nitropyridine can be obtained via the synthesis of 2,6-dibromopyridine followed by nitration and debromination.
Sulfonation of pyridine is even more difficult than nitration. However, pyridine-3-sulfonic acid can be obtained. Reaction with the SO3 group also facilitates addition of sulfur to the nitrogen atom, especially in the presence of a mercury(II) sulfate catalyst.
In contrast to the sluggish nitrations and sulfonations, the bromination and chlorination reaction, chlorination of pyridine proceed well.

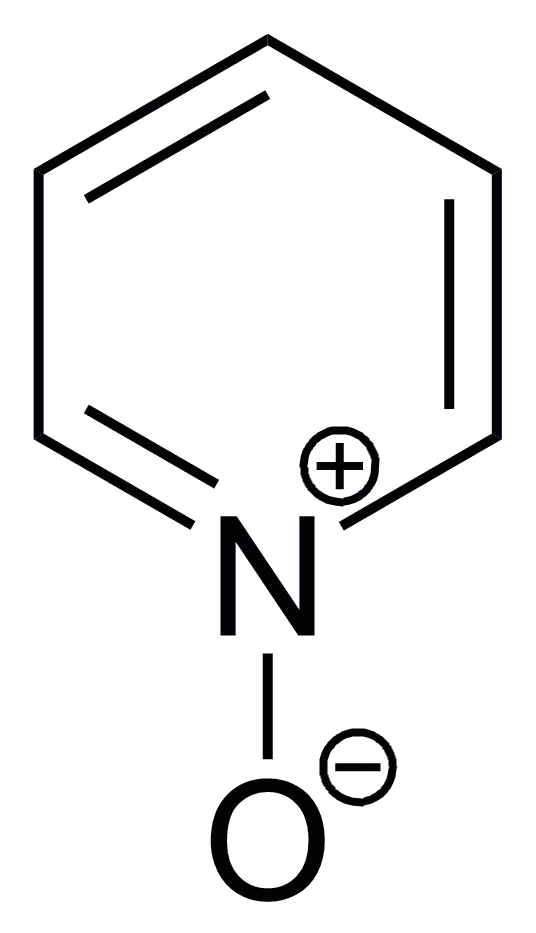 Oxidation of pyridine occurs at nitrogen to give pyridine ''N''-oxide. The oxidation can be achieved with peracids:
:C5H5N + RCO3H → C5H5NO + RCO2H
Some electrophilic substitutions on the pyridine are usefully effected using pyridine ''N''-oxide followed by deoxygenation. Addition of oxygen suppresses further reactions at nitrogen atom and promotes substitution at the 2- and 4-carbons. The oxygen atom can then be removed, e.g. using zinc dust.
Oxidation of pyridine occurs at nitrogen to give pyridine ''N''-oxide. The oxidation can be achieved with peracids:
:C5H5N + RCO3H → C5H5NO + RCO2H
Some electrophilic substitutions on the pyridine are usefully effected using pyridine ''N''-oxide followed by deoxygenation. Addition of oxygen suppresses further reactions at nitrogen atom and promotes substitution at the 2- and 4-carbons. The oxygen atom can then be removed, e.g. using zinc dust.


 Many nucleophilic substitutions occur more easily not with bare pyridine but with pyridine modified with bromine, chlorine, fluorine, or sulfonic acid fragments that then become a leaving group. So fluorine is the best leaving group for the substitution with organolithium compounds. The nucleophilic attack compounds may be alkoxides, thiolates, amines, and ammonia (at elevated pressures).
In general, the hydride ion is a poor leaving group and occurs only in a few heterocyclic reactions. They include the Chichibabin reaction, which yields pyridine derivatives Amination, aminated at the 2-position. Here, sodium amide is used as the nucleophile yielding 2-aminopyridine. The hydride ion released in this reaction combines with a proton of an available amino group, forming a hydrogen molecule.
Analogous to benzene, nucleophilic substitutions to pyridine can result in the formation of pyridyne intermediates as heteroaryne. For this purpose, pyridine derivatives can be eliminated with good leaving groups using strong bases such as sodium and potassium tert-butoxide. The subsequent addition of a nucleophile to the triple bond has low selectivity, and the result is a mixture of the two possible adducts.
Many nucleophilic substitutions occur more easily not with bare pyridine but with pyridine modified with bromine, chlorine, fluorine, or sulfonic acid fragments that then become a leaving group. So fluorine is the best leaving group for the substitution with organolithium compounds. The nucleophilic attack compounds may be alkoxides, thiolates, amines, and ammonia (at elevated pressures).
In general, the hydride ion is a poor leaving group and occurs only in a few heterocyclic reactions. They include the Chichibabin reaction, which yields pyridine derivatives Amination, aminated at the 2-position. Here, sodium amide is used as the nucleophile yielding 2-aminopyridine. The hydride ion released in this reaction combines with a proton of an available amino group, forming a hydrogen molecule.
Analogous to benzene, nucleophilic substitutions to pyridine can result in the formation of pyridyne intermediates as heteroaryne. For this purpose, pyridine derivatives can be eliminated with good leaving groups using strong bases such as sodium and potassium tert-butoxide. The subsequent addition of a nucleophile to the triple bond has low selectivity, and the result is a mixture of the two possible adducts.
 Lewis acids easily add to the nitrogen atom of pyridine, forming pyridinium salts. The reaction with alkyl halides leads to alkylation of the nitrogen atom. This creates a positive charge in the ring that increases the reactivity of pyridine to both oxidation and reduction. The Zincke reaction is used for the selective introduction of radicals in pyridinium compounds (it has no relation to the chemical element zinc).
Lewis acids easily add to the nitrogen atom of pyridine, forming pyridinium salts. The reaction with alkyl halides leads to alkylation of the nitrogen atom. This creates a positive charge in the ring that increases the reactivity of pyridine to both oxidation and reduction. The Zincke reaction is used for the selective introduction of radicals in pyridinium compounds (it has no relation to the chemical element zinc).
 Piperidine is produced by hydrogenation of pyridine with a nickel-, cobalt-, or ruthenium-based catalyst at elevated temperatures. The hydrogenation of pyridine to piperidine releases 193.8 kJ·mol−1, which is slightly less than the energy of the hydrogenation of
Piperidine is produced by hydrogenation of pyridine with a nickel-, cobalt-, or ruthenium-based catalyst at elevated temperatures. The hydrogenation of pyridine to piperidine releases 193.8 kJ·mol−1, which is slightly less than the energy of the hydrogenation of
 Transition metal pyridine complexes are numerous.
Typical octahedral complexes have the stoichiometry and . Octahedral homoleptic complexes of the type are rare or tend to dissociate pyridine. Numerous square planar complexes are known, such as Crabtree's catalyst. The pyridine ligand replaced during the reaction is restored after its completion.
The ''η''6 coordination mode, as occurs in ''η''6 benzene complexes, is observed only in Steric effects, sterically encumbered derivatives that block the nitrogen center.
Transition metal pyridine complexes are numerous.
Typical octahedral complexes have the stoichiometry and . Octahedral homoleptic complexes of the type are rare or tend to dissociate pyridine. Numerous square planar complexes are known, such as Crabtree's catalyst. The pyridine ligand replaced during the reaction is restored after its completion.
The ''η''6 coordination mode, as occurs in ''η''6 benzene complexes, is observed only in Steric effects, sterically encumbered derivatives that block the nitrogen center.
 It is also used in the textile industry to improve network capacity of cotton.
It is also used in the textile industry to improve network capacity of cotton.

 As a base, pyridine can be used as the Karl Fischer titration, Karl Fischer reagent, but it is usually replaced by alternatives with a more pleasant odor, such as imidazole.
Pyridinium chlorochromate, Cornforth reagent, pyridinium dichromate, and the Collins reagent (the complex of chromium trioxide, chromium(VI) oxide) are used for the oxidation of alcohols.
As a base, pyridine can be used as the Karl Fischer titration, Karl Fischer reagent, but it is usually replaced by alternatives with a more pleasant odor, such as imidazole.
Pyridinium chlorochromate, Cornforth reagent, pyridinium dichromate, and the Collins reagent (the complex of chromium trioxide, chromium(VI) oxide) are used for the oxidation of alcohols.
 Exposure to pyridine would normally lead to its inhalation and absorption in the lungs and gastrointestinal tract, where it either remains unchanged or is metabolism, metabolized. The major products of pyridine metabolism are ''N''-methylpyridiniumhydroxide, which are formed by N-methyltransferase, ''N''-methyltransferases (e.g., pyridine N-methyltransferase, pyridine ''N''-methyltransferase), as well as pyridine ''N''-oxide, and 2-, 3-, and 4-hydroxypyridine, which are generated by the action of monooxygenase. In humans, pyridine is metabolized only into ''N''-methylpyridiniumhydroxide.
Exposure to pyridine would normally lead to its inhalation and absorption in the lungs and gastrointestinal tract, where it either remains unchanged or is metabolism, metabolized. The major products of pyridine metabolism are ''N''-methylpyridiniumhydroxide, which are formed by N-methyltransferase, ''N''-methyltransferases (e.g., pyridine N-methyltransferase, pyridine ''N''-methyltransferase), as well as pyridine ''N''-oxide, and 2-, 3-, and 4-hydroxypyridine, which are generated by the action of monooxygenase. In humans, pyridine is metabolized only into ''N''-methylpyridiniumhydroxide.
Synthesis and properties of pyridines
at chemsynthesis.com
Synthesis of pyridines (overview of recent methods)
{{Authority control Pyridines, Amine solvents Foul-smelling chemicals Aromatic bases Simple aromatic rings Functional groups Aromatic solvents
basic
BASIC (Beginners' All-purpose Symbolic Instruction Code) is a family of general-purpose, high-level programming languages designed for ease of use. The original version was created by John G. Kemeny and Thomas E. Kurtz at Dartmouth College ...
heterocyclic
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and ...
organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The ...
with the chemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
. It is structurally related to benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
, with one methine group
In organic chemistry, a methine group or methine bridge is a trivalent functional group , derived formally from methane. It consists of a carbon atom bound by two single bonds and one double bond, where one of the single bonds is to a hydrogen. T ...
replaced by a nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
atom. It is a highly flammable, weakly alkali
In chemistry, an alkali (; from ar, القلوي, al-qaly, lit=ashes of the saltwort) is a basic, ionic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a ...
ne, water-miscible liquid with a distinctive, unpleasant fish-like smell. Pyridine is colorless, but older or impure samples can appear yellow, due to the formation of extended, unsaturated polymeric chains, which show significant electrical conductivity. The pyridine ring occurs in many important compounds, including agrochemical
An agrochemical or agrichemical, a contraction of ''agricultural chemical'', is a chemical product used in industrial agriculture. Agrichemical refers to biocides ( pesticides including insecticides, herbicides, fungicides and nematicides) an ...
s, pharmaceutical
A medication (also called medicament, medicine, pharmaceutical drug, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy (pharmacotherapy) is an important part of the medical field and re ...
s, and vitamin
A vitamin is an organic molecule (or a set of molecules closely related chemically, i.e. vitamers) that is an Nutrient#Essential nutrients, essential micronutrient that an organism needs in small quantities for the proper functioning of its ...
s. Historically, pyridine was produced from coal tar
Coal tar is a thick dark liquid which is a by-product of the production of coke and coal gas from coal. It is a type of creosote. It has both medical and industrial uses. Medicinally it is a topical medication applied to skin to treat psoriasi ...
. As of 2016, it is synthesized on the scale of about 20,000 tons per year worldwide.
Properties
Physical properties
 The molecular
The molecular electric dipole moment
The electric dipole moment is a measure of the separation of positive and negative electrical charges within a system, that is, a measure of the system's overall polarity. The SI unit for electric dipole moment is the coulomb-meter (C⋅m). The ...
is 2.2 debye
The debye (symbol: D) (; ) is a CGS unit (a non- SI metric unit) of electric dipole momentTwo equal and opposite charges separated by some distance constitute an electric dipole. This dipole possesses an electric dipole moment whose value is give ...
s. Pyridine is diamagnetic
Diamagnetic materials are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagnetic materials are attracted ...
and has a diamagnetic susceptibility of −48.7 × 10−6 cm3·mol−1. The standard enthalpy of formation
In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent elements in their reference state, wi ...
is 100.2 kJ·mol−1 in the liquid phase Lide, p. 5-28 and 140.4 kJ·mol−1 in the gas phase. At 25 °C pyridine has a viscosity
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water.
Viscosity quantifies the inte ...
of 0.88 mPa/s and thermal conductivity
The thermal conductivity of a material is a measure of its ability to conduct heat. It is commonly denoted by k, \lambda, or \kappa.
Heat transfer occurs at a lower rate in materials of low thermal conductivity than in materials of high thermal ...
of 0.166 W·m−1·K−1. The enthalpy of vaporization
The enthalpy of vaporization (symbol ), also known as the (latent) heat of vaporization or heat of evaporation, is the amount of energy (enthalpy) that must be added to a liquid substance to transform a quantity of that substance into a gas. T ...
is 35.09 kJ·mol−1 at the boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envir ...
and normal pressure. The enthalpy of fusion
In thermodynamics, the enthalpy of fusion of a substance, also known as (latent) heat of fusion, is the change in its enthalpy resulting from providing energy, typically heat, to a specific quantity of the substance to change its state from a s ...
is 8.28 kJ·mol−1 at the melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends ...
.
The critical parameters of pyridine are pressure 6.70 MPa, temperature 620 K and volume 229 cm3·mol−1. In the temperature range 340–426 °C its vapor pressure ''p'' can be described with the Antoine equation
The Antoine equation is a class of semi-empirical correlations describing the relation between vapor pressure and temperature for pure substances. The Antoine equation is derived from the Clausius–Clapeyron relation. The equation was presented ...
:
where ''T'' is temperature, ''A'' = 4.16272, ''B'' = 1371.358 K and ''C'' = −58.496 K.
Structure
Pyridine ring forms a hexagon. Slight variations of the and distances as well as the bond angles are observed.Crystallography
Pyridine crystallizes in anorthorhombic crystal system
In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic lattices result from stretching a cubic lattice along two of its orthogonal pairs by two different factors, resulting in a rectangular prism with a r ...
with space group
In mathematics, physics and chemistry, a space group is the symmetry group of an object in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of an object that leave it unchan ...
''Pna21'' and lattice parameters ''a'' = 1752 pm, ''b'' = 897 pm, ''c'' = 1135 pm, and 16 formula unit
In chemistry, a formula unit is the empirical formula of any ionic or covalent network solid compound used as an independent entity for stoichiometric calculations. It is the lowest whole number ratio of ions represented in an ionic compound. Exa ...
s per unit cell
In geometry, biology, mineralogy and solid state physics, a unit cell is a repeating unit formed by the vectors spanning the points of a lattice. Despite its suggestive name, the unit cell (unlike a unit vector, for example) does not necessaril ...
(measured at 153 K). For comparison, crystalline benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
is also orthorhombic, with space group ''Pbca'', ''a'' = 729.2 pm, ''b'' = 947.1 pm, ''c'' = 674.2 pm (at 78 K), but the number of molecules per cell is only 4. This difference is partly related to the lower symmetry of the individual pyridine molecule (C2v vs D6h for benzene). A trihydrate
In chemistry, a hydrate is a substance that contains water or its constituent elements. The chemical state of the water varies widely between different classes of hydrates, some of which were so labeled before their chemical structure was understo ...
(pyridine·3H2O) is known; it also crystallizes in an orthorhombic system in the space group ''Pbca'', lattice parameters ''a'' = 1244 pm, ''b'' = 1783 pm, ''c'' = 679 pm and eight formula units per unit cell (measured at 223 K).
Spectroscopy
The optical absorption spectrum of pyridine inhexane
Hexane () is an organic compound, a straight-chain alkane with six carbon atoms and has the molecular formula C6H14.
It is a colorless liquid, odorless when pure, and with boiling points approximately . It is widely used as a cheap, relatively ...
consists of bands at the wavelength
In physics, the wavelength is the spatial period of a periodic wave—the distance over which the wave's shape repeats.
It is the distance between consecutive corresponding points of the same phase on the wave, such as two adjacent crests, tro ...
s of 195, 251, and 270 nm. With respective extinction coefficients (''ε'') of 7500, 2000, and 450 L·mol−1·cm−1, these bands are assigned to π → π*, π → π*, and n → π* transitions.
The 1H nuclear magnetic resonance
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with a ...
(NMR) spectrum shows signals for α-( δ 8.5), γ-(δ7.5) and β-protons (δ7). By contrast, the proton signal for benzene is found at δ7.27. The larger chemical shifts of the α- and γ-protons in comparison to benzene result from the lower electron density in the α- and γ-positions, which can be derived from the resonance structures. The situation is rather similar for the 13C NMR spectra of pyridine and benzene: pyridine shows a triplet at ''δ''(α-C) = 150 ppm, δ(β-C) = 124 ppm and δ(γ-C) = 136 ppm, whereas benzene has a single line at 129 ppm. All shifts are quoted for the solvent-free substances. Pyridine is conventionally detected by the gas chromatography
Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, ...
and mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is use ...
methods.
Bonding
 Pyridine has a conjugated system of six π electrons that are delocalized over the ring. The molecule is planar and, thus, follows the Hückel criteria for aromatic systems. In contrast to benzene, the
Pyridine has a conjugated system of six π electrons that are delocalized over the ring. The molecule is planar and, thus, follows the Hückel criteria for aromatic systems. In contrast to benzene, the electron density
In quantum chemistry, electron density or electronic density is the measure of the probability of an electron being present at an infinitesimal element of space surrounding any given point. It is a scalar quantity depending upon three spatial va ...
is not evenly distributed over the ring, reflecting the negative inductive effect
In chemistry, the inductive effect in a molecule is a local change in the electron density due to electron-withdrawing or electron-donating groups elsewhere in the molecule, resulting in a permanent dipole in a bond.
It is present in a σ (sigm ...
of the nitrogen atom. For this reason, pyridine has a dipole moment and a weaker resonant stabilization than benzene ( resonance energy 117 kJ·mol−1 in pyridine vs. 150 kJ·mol−1 in benzene).
The ring atoms in the pyridine molecule are sp2-hybridized. The nitrogen is involved in the π-bonding aromatic system using its unhybridized p orbital. The lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
is in an sp2 orbital, projecting outward from the ring in the same plane as the σ bond
In chemistry, sigma bonds (σ bonds) are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most simply defined for diatomic molecules using the language and tools of sy ...
s. As a result, the lone pair does not contribute to the aromatic system but importantly influences the chemical properties of pyridine, as it easily supports bond formation via an electrophilic attack. However, because of the separation of the lone pair from the aromatic ring system, the nitrogen atom cannot exhibit a positive mesomeric effect
Mesomeric Effect in Organic Chemistry
The Mesomeric Effect
The mesomeric effect (or resonance effect) in chemistry is a property of substituents or functional groups in a chemical compound. It is defined as the polarity produced in the molecu ...
.
Many analogues of pyridine are known where N is replaced by other heteroatoms (see figure below). Substitution of one C–H in pyridine with a second N gives rise to the diazine
In organic chemistry, diazines are a group of organic compounds having the molecular formula . Each contains a benzene ring in which two of the C-H fragments have been replaced by isolobal nitrogen. There are three structural isomers:
* pyridaz ...
heterocycles (C4H4N2), with the names pyridazine
Pyridazine is an aromatic, heterocyclic, organic compound with the molecular formula . It contains a six-membered ring with two adjacent nitrogen atoms. It is a colorless liquid with a boiling point of 208 °C. It is isomeric with two other ...
, pyrimidine
Pyrimidine (; ) is an aromatic, heterocyclic, organic compound similar to pyridine (). One of the three diazines (six-membered heterocyclics with two nitrogen atoms in the ring), it has nitrogen atoms at positions 1 and 3 in the ring. The other ...
, and pyrazine
Pyrazine is a heterocyclic aromatic organic compound with the chemical formula C4H4N2. It is a symmetrical molecule with point group D2h. Pyrazine is less basic than pyridine, pyridazine and pyrimidine. It is a ''"deliquescent crystal or wax-lik ...
.
History
 Impure pyridine was undoubtedly prepared by early
Impure pyridine was undoubtedly prepared by early alchemists
Alchemy (from Arabic: ''al-kīmiyā''; from Ancient Greek: χυμεία, ''khumeía'') is an ancient branch of natural philosophy, a philosophical and protoscientific tradition that was historically practiced in China, India, the Muslim world, ...
by heating animal bones and other organic matter, but the earliest documented reference is attributed to the Scottish scientist Thomas Anderson. In 1849, Anderson examined the contents of the oil obtained through high-temperature heating of animal bones. Among other substances, he separated from the oil a colorless liquid with unpleasant odor, from which he isolated pure pyridine two years later. He described it as highly soluble in water, readily soluble in concentrated acids and salts upon heating, and only slightly soluble in oils.
Owing to its flammability, Anderson named the new substance ''pyridine'', after gr, πῦρ (pyr) meaning ''fire''. The suffix '' idine'' was added in compliance with the chemical nomenclature, as in ''toluidine
There are three isomers of toluidine, which are organic compounds. These isomers are ''o''-toluidine, ''m''-toluidine, and ''p''-toluidine, with the prefixed letter abbreviating, respectively, ''ortho''; ''meta''; and ''para''. All three are aryl ...
'', to indicate a cyclic compound
A cyclic compound (or ring compound) is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where al ...
containing a nitrogen atom.
The chemical structure of pyridine was determined decades after its discovery. Wilhelm Körner
Wilhelm Körner, later a.k.a. Guglielmo Körner (April 20, 1839 in Cassel – March 29, 1925 in Milan), was a German chemist.
Life
Körner studied chemistry at Giessen, where he graduated in 1860. In 1866, he became assistant to Kekulé at ...
(1869) and James Dewar
Sir James Dewar (20 September 1842 – 27 March 1923) was a British chemist and physicist. He is best known for his invention of the vacuum flask, which he used in conjunction with research into the liquefaction of gases. He also studied ato ...
(1871) suggested that, in analogy between quinoline
Quinoline is a heterocyclic aromatic organic compound with the chemical formula C9H7N. It is a colorless hygroscopic liquid with a strong odor. Aged samples, especially if exposed to light, become yellow and later brown. Quinoline is only sli ...
and naphthalene
Naphthalene is an organic compound with formula . It is the simplest polycyclic aromatic hydrocarbon, and is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromati ...
, the structure of pyridine is derived from benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
by substituting one C–H unit with a nitrogen atom. The suggestion by Körner and Dewar was later confirmed in an experiment where pyridine was reduced to piperidine
Piperidine is an organic compound with the molecular formula (CH2)5NH. This heterocyclic compound, heterocyclic amine consists of a six-membered ring containing five methylene bridges (–CH2–) and one amine bridge (–NH–). It is a colorless ...
with sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable iso ...
in ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl ...
. In 1876, William Ramsay
Sir William Ramsay (; 2 October 1852 – 23 July 1916) was a Scottish chemist who discovered the noble gases and received the Nobel Prize in Chemistry in 1904 "in recognition of his services in the discovery of the inert gaseous elements ...
combined acetylene
Acetylene (systematic name: ethyne) is the chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure ...
and hydrogen cyanide
Hydrogen cyanide, sometimes called prussic acid, is a chemical compound with the formula HCN and structure . It is a colorless, extremely poisonous, and flammable liquid that boils slightly above room temperature, at . HCN is produced on an ...
into pyridine in a red-hot iron-tube furnace. This was the first synthesis of a heteroaromatic compound.
The first major synthesis of pyridine derivatives was described in 1881 by Arthur Rudolf Hantzsch
Arthur Rudolf Hantzsch (7 March 1857 – 14 March 1935) was a German chemist.
Life and work
Hantzsch studied chemistry in Dresden and graduated at the University of Würzburg under Johannes Wislicenus. As a professor, he taught at the Universitie ...
. The Hantzsch pyridine synthesis
The Hantzsch pyridine synthesis or Hantzsch dihydropyridine synthesis is a multi-component organic reaction between an aldehyde such as formaldehyde, 2 equivalents of a β-keto ester such as ethyl acetoacetate and a nitrogen donor such as ammoni ...
typically uses a 2:1:1 mixture of a β-keto acid
In organic chemistry, keto acids or ketoacids (also called oxo acids or oxoacids) are organic compounds that contain a carboxylic acid group () and a ketone group ().Franz Dietrich Klingler, Wolfgang Ebertz "Oxocarboxylic Acids" in Ullmann's E ...
(often acetoacetate
Acetoacetic acid (also acetoacetate and diacetic acid) is the organic compound with the formula CHCOCHCOOH. It is the simplest beta-keto acid, and like other members of this class, it is unstable. The methyl and ethyl esters, which are quite stab ...
), an aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
(often formaldehyde
Formaldehyde ( , ) (systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section F ...
), and ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous was ...
or its salt as the nitrogen donor. First, a double hydrogenated
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic co ...
pyridine is obtained, which is then oxidized to the corresponding pyridine derivative. Emil Knoevenagel
Heinrich Emil Albert Knoevenagel (18 June 1865 – 11 August 1921) was the German chemist who established the Knoevenagel condensation reaction. The Knoevenagel condensation reaction of benzaldehydes with nitroalkanes is a classic general met ...
showed that asymmetrically substituted pyridine derivatives can be produced with this process.
Aleksei Chichibabin
Alekséy Yevgényevich Chichibábin (russian: Алексей Евгеньевич Чичибабин) was a Soviet Union, Soviet/Russian organic chemist, born , Kuzemin village, current Sumy Oblast, Ukraine, died in Paris, France, 15 August 1945. H ...
invented a pyridine synthesis reaction, which was based on inexpensive reagents. This method is still used for the industrial production of pyridine.
Occurrence
Pyridine is not abundant in nature, except for the leaves and roots of belladonna (''Atropa belladonna
''Atropa belladonna'', commonly known as belladonna or deadly nightshade, is a toxic perennial herbaceous plant in the nightshade family Solanaceae, which also includes tomatoes, potatoes, and eggplant (aubergine). It is native to Europe, North ...
'') and in marshmallow (''Althaea officinalis
''Althaea officinalis'', the marsh mallow or marshmallow, is a species of flowering plant indigenous to Europe, Western Asia and North Africa, which is used in herbalism and as an ornamental plant. A confection made from the root since ancient ...
''). Pyridine derivatives, however, are often part of biomolecules such as alkaloid
Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar ...
s.
In daily life, trace amounts of pyridine are components of the volatile organic compound
Volatile organic compounds (VOCs) are organic compounds that have a high vapour pressure at room temperature. High vapor pressure correlates with a low boiling point, which relates to the number of the sample's molecules in the surrounding air, a ...
s that are produced in roasting and canning
Canning is a method of food preservation in which food is processed and sealed in an airtight container (jars like Mason jars, and steel and tin cans). Canning provides a shelf life that typically ranges from one to five years, although u ...
processes, e.g. in fried chicken, sukiyaki
is a Japanese dish that is prepared and served in the ''nabemono'' (Japanese hot pot) style.
It consists of meat (usually thinly sliced beef) which is slowly cooked or simmered at the table, alongside vegetables and other ingredients, in ...
, roasted coffee, potato chips, and fried bacon
Bacon is a type of salt-cured pork made from various cuts, typically the belly or less fatty parts of the back. It is eaten as a side dish (particularly in breakfasts), used as a central ingredient (e.g., the bacon, lettuce, and tomato sand ...
. Traces of pyridine can be found in Beaufort cheese
Beaufort () is a firm, raw cow's milk cheese associated with the gruyère family. An Alpine cheese, it is produced in Beaufortain, Tarentaise valley and Maurienne, which are located in the Savoie region of the French Alps.
Varieties
There are ...
, vaginal secretion
Vaginal discharge is a mixture of liquid, cells, and bacteria that lubricate and protect the vagina. This mixture is constantly produced by the cells of the vagina and cervix, and it exits the body through the vaginal opening. The composition, amo ...
s, black tea
Black tea, also translated to red tea in various East Asian languages, is a type of tea that is more oxidized than oolong, yellow, white and green teas. Black tea is generally stronger in flavour than other teas. All five types are made from ...
, saliva of those suffering from gingivitis
Gingivitis is a non-destructive disease that causes inflammation of the gums. The most common form of gingivitis, and the most common form of periodontal disease overall, is in response to bacterial biofilms (also called plaque) that is attached ...
, and sunflower honey.
bipyridine Bipyridines also known as bipyridyls, dipyridyls, and dipyridines, are a family of chemical compounds with the formula (C5H4N)2, consisting of two pyridyl (C5H4N) rings. Pyridine is an aromatic nitrogen-containing heterocycle. Bipyridines are of sig ...
File:Dipicolinic acid.svg, pyridine-2,6-dicarboxylic acid (dipicolinic acid
Dipicolinic acid (pyridine-2,6-dicarboxylic acid or PDC and DPA) is a chemical compound which plays a role in the heat resistance of bacterial endospores. It is also used to prepare dipicolinato ligated lanthanide and transition metal complexes f ...
)
File:PyridiniumVerbindungen.svg, General form of the pyridinium
Pyridinium refers to the cation . It is the conjugate acid of pyridine. Many related cations are known involving substituted pyridines, e.g. picolines, lutidines, collidines. They are prepared by treating pyridine with acids.
As pyridine is oft ...
cation
Production
Historically, pyridine was extracted fromcoal tar
Coal tar is a thick dark liquid which is a by-product of the production of coke and coal gas from coal. It is a type of creosote. It has both medical and industrial uses. Medicinally it is a topical medication applied to skin to treat psoriasi ...
or obtained as a byproduct of coal gasification
Gasification is a process that converts biomass- or fossil fuel-based carbonaceous materials into gases, including as the largest fractions: nitrogen (N2), carbon monoxide (CO), hydrogen (H2), and carbon dioxide (). This is achieved by reacting ...
. The process is labor-consuming and inefficient: coal tar
Coal tar is a thick dark liquid which is a by-product of the production of coke and coal gas from coal. It is a type of creosote. It has both medical and industrial uses. Medicinally it is a topical medication applied to skin to treat psoriasi ...
contains only about 0.1% pyridine, and therefore a multi-stage purification was required, which further reduced the output. Nowadays, most pyridines are synthesized from ammonia, aldehydes, and nitriles, a few combinations of which are suited for pyridine itself. Various name reaction A name reaction is a chemical reaction named after its discoverers or developers. Among the tens of thousands of organic reactions that are known, hundreds of such reactions are well-known enough to be named after people. Well-known examples include ...
s are also known, but they are not practiced on scale.
In 1989, 26,000 tonnes of pyridine was produced worldwide. Other major derivatives are 2-, 3-, 4-methylpyridine
4-Methylpyridine is the organic compound with the formula CH3C5H4N. It is one of the three isomers of methylpyridine. This pungent liquid is a building block for the synthesis of other heterocyclic compounds. Its conjugate acid, the 4-methylpyrid ...
s and 5-ethyl-2-methylpyridine
5-Ethyl-2-methylpyridine is an organic compound with the formula (C2H5)(CH3)C5H3N. One of several isomeric pyridines with this formula, this derivative is of interest because it is efficiently prepared from simple reagents and it is a convenient ...
. The combined scale of these alkylpyridines matches that of pyridine itself. Among the largest 25 production sites for pyridine, eleven are located in Europe (as of 1999). The major producers of pyridine include Evonik Industries
Evonik Industries AG is a stock-listed German specialty chemicals company headquartered in Essen, North Rhine-Westphalia, Germany. It is the second largest chemicals company in Germany, and one of the largest specialty chemicals companies in the ...
, Rütgers Chemicals, Jubilant Life Sciences, Imperial Chemical Industries
Imperial Chemical Industries (ICI) was a British chemical company. It was, for much of its history, the largest manufacturer in Britain.
It was formed by the merger of four leading British chemical companies in 1926.
Its headquarters were at M ...
, and Koei Chemical. Pyridine production significantly increased in the early 2000s, with an annual production capacity of 30,000 tonnes in mainland China alone. The US–Chinese joint venture Vertellus is currently the world leader in pyridine production.
Chichibabin synthesis
TheChichibabin pyridine synthesis
The Chichibabin pyridine synthesis () is a method for synthesizing pyridine rings. The reaction involves the condensation reaction of aldehydes, ketones, α,β-Unsaturated carbonyl compounds, or any combination of the above, with ammonia. It was ...
was reported in 1924 and the basic approach underpins several industrial routes. In its general form, the reaction involves the condensation reaction
In organic chemistry, a condensation reaction is a type of chemical reaction in which two molecules are combined to form a single molecule, usually with the loss of a small molecule such as water. If water is lost, the reaction is also known as a ...
of aldehydes
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group ...
, ketones, α,β-Unsaturated carbonyl compound, α,β-unsaturated carbonyl compounds, or any combination of the above, in ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous was ...
or amine, ammonia derivatives. Application of the Chichibabin pyridine synthesis suffer from low yields, often about 30%, however the precursors are inexpensive. In particular, unsubstituted pyridine is produced from formaldehyde
Formaldehyde ( , ) (systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section F ...
and acetaldehyde. First, acrolein is formed in a Knoevenagel condensation from the acetaldehyde and formaldehyde. The acrolein then condensation reaction, condenses with acetaldehyde and ammonia to give dihydropyridine, which is oxidized to pyridine. This process is carried out in a gas phase at 400–450 °C. Typical catalysts are modified forms of alumina and silica. The reaction has been tailored to produce various methylpyridines.
Dealkylation and decarboxylation of substituted pyridines
Pyridine can be prepared by dealkylation of alkylated pyridines, which are obtained as byproducts in the syntheses of other pyridines. The oxidative dealkylation is carried out either using air over vanadium(V) oxide catalyst, by vapor-dealkylation on nickel-based catalyst, or hydrodealkylation with a silver- or platinum-based catalyst. Yields of pyridine up to be 93% can be achieved with the nickel-based catalyst. Pyridine can also be produced by the decarboxylation of nicotinic acid with copper chromite.Bönnemann cyclization
 The trimerization of a part of a nitrile molecule and two parts of
The trimerization of a part of a nitrile molecule and two parts of acetylene
Acetylene (systematic name: ethyne) is the chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure ...
into pyridine is called Bönnemann cyclization. This modification of the Walter Reppe, Reppe synthesis can be activated either by heat or by Photochemistry, light. While the thermal activation requires high pressures and temperatures, the photoinduced cycloaddition proceeds at ambient conditions with CoCp2(cod) (Cp = cyclopentadienyl, cod = 1,5-cyclooctadiene) as a catalyst, and can be performed even in water. A series of pyridine derivatives can be produced in this way. When using acetonitrile as the nitrile, 2-methylpyridine is obtained, which can be dealkylated to pyridine.
Other methods
The Kröhnke pyridine synthesis provides a fairly general method for generating substituted pyridines using pyridine itself as a reagent which does not become incorporated into the final product. The reaction of pyridine with bromomethyl ketones gives the relatedpyridinium
Pyridinium refers to the cation . It is the conjugate acid of pyridine. Many related cations are known involving substituted pyridines, e.g. picolines, lutidines, collidines. They are prepared by treating pyridine with acids.
As pyridine is oft ...
salt, wherein the methylene group is highly acidic. This species undergoes a Michael addition, Michael-like addition to α,β-unsaturated carbonyls in the presence of ammonium acetate to undergo ring closure and formation of the targeted substituted pyridine as well as pyridinium bromide.
 The Ciamician–Dennstedt rearrangement entails the ring-expansion of pyrrole with dichlorocarbene to 3-Chloropyridine, 3-chloropyridine.
The Ciamician–Dennstedt rearrangement entails the ring-expansion of pyrrole with dichlorocarbene to 3-Chloropyridine, 3-chloropyridine.
 In the Gattermann–Skita synthesis, a Malonic ester synthesis, malonate ester salt reacts with dichloromethylamine.
In the Gattermann–Skita synthesis, a Malonic ester synthesis, malonate ester salt reacts with dichloromethylamine.
 Other methods include the Boger pyridine synthesis and Diels–Alder reaction of an alkene and an oxazole.
Other methods include the Boger pyridine synthesis and Diels–Alder reaction of an alkene and an oxazole.
Biosynthesis
Several pyridine derivatives play important roles in biological systems. While its biosynthesis is not fully understood, nicotinic acid (vitamin B3) occurs in some bacteria, fungi, and mammals. Mammals synthesize nicotinic acid through oxidation of the amino acid tryptophan, where an intermediate product, the aniline derivative kynurenine, creates a pyridine derivative, quinolinate and then nicotinic acid. On the contrary, the bacteria ''Mycobacterium tuberculosis'' and ''Escherichia coli'' produce nicotinic acid by condensation of glyceraldehyde 3-phosphate and aspartic acid.Reactions
Because of the electronegativenitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
in the pyridine ring, therefore pyridine enters less readily into electrophilic aromatic substitution reactions than benzene derivatives. Instead, in terms of its reactivity, pyridine resembles nitrobenzene.
Correspondingly pyridine is more prone to nucleophilic substitution, as evidenced by the ease of metalation by strong organometallic bases. The reactivity of pyridine can be distinguished for three chemical groups. With electrophiles, electrophilic substitution takes place where pyridine expresses aromatic properties. With nucleophiles, pyridine reacts at positions 2 and 4 and thus behaves similar to imines and carbonyls. The reaction with many Lewis acids results in the addition to the nitrogen atom of pyridine, which is similar to the reactivity of tertiary amines. The ability of pyridine and its derivatives to oxidize, forming amine oxides (''N''-oxides), is also a feature of tertiary amines.
The nitrogen center of pyridine features a basic lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
of electrons. This lone pair does not overlap with the aromatic π-system ring, consequently pyridine is Base (chemistry), basic, having chemical properties similar to those of tertiary amines. Protonation gives pyridinium
Pyridinium refers to the cation . It is the conjugate acid of pyridine. Many related cations are known involving substituted pyridines, e.g. picolines, lutidines, collidines. They are prepared by treating pyridine with acids.
As pyridine is oft ...
, C5H5NH+.The pKa, p''K''a of the conjugate acid (the pyridinium cation) is 5.25. The structures of pyridine and pyridinium are almost identical. The pyridinium cation is isoelectronic with benzene. Pyridinium ''p''-toluenesulfonic acid, toluenesulfonate (PPTS) is an illustrative pyridinium salt; it is produced by treating pyridine with P-Toluenesulfonic acid, ''p''-toluenesulfonic acid. In addition to protonation, pyridine undergoes N-centred alkylation, acylation, and N-oxidation, ''N''-oxidation. Pyridine and poly(4-vinyl) pyridine have been shown to form conducting molecular wires with remarkable polyenimine structure on UV irradiation, a process which accounts for at least some of the visible light absorption by aged pyridine samples. These wires have been theoretically predicted to be both highly efficient electron donors and acceptors, and yet are resistant to air oxidation.
Electrophilic substitutions
Owing to the decreased electron density in the aromatic system, electrophilic substitutions are suppressed in pyridine and its derivatives. Friedel–Crafts reaction, Friedel–Crafts alkylation or acylation, usually fail for pyridine because they lead only to the addition at the nitrogen atom. Substitutions usually occur at the 3-position, which is the most electron-rich carbon atom in the ring and is, therefore, more susceptible to an electrophilic addition.

 Direct nitration of pyridine is sluggish. Pyridine derivatives wherein the nitrogen atom is screened sterically and/or electronically can be obtained by nitration with nitronium tetrafluoroborate (NO2BF4). In this way, 3-nitropyridine can be obtained via the synthesis of 2,6-dibromopyridine followed by nitration and debromination.
Sulfonation of pyridine is even more difficult than nitration. However, pyridine-3-sulfonic acid can be obtained. Reaction with the SO3 group also facilitates addition of sulfur to the nitrogen atom, especially in the presence of a mercury(II) sulfate catalyst.
In contrast to the sluggish nitrations and sulfonations, the bromination and chlorination reaction, chlorination of pyridine proceed well.
Direct nitration of pyridine is sluggish. Pyridine derivatives wherein the nitrogen atom is screened sterically and/or electronically can be obtained by nitration with nitronium tetrafluoroborate (NO2BF4). In this way, 3-nitropyridine can be obtained via the synthesis of 2,6-dibromopyridine followed by nitration and debromination.
Sulfonation of pyridine is even more difficult than nitration. However, pyridine-3-sulfonic acid can be obtained. Reaction with the SO3 group also facilitates addition of sulfur to the nitrogen atom, especially in the presence of a mercury(II) sulfate catalyst.
In contrast to the sluggish nitrations and sulfonations, the bromination and chlorination reaction, chlorination of pyridine proceed well.

Pyridine ''N''-oxide
 Oxidation of pyridine occurs at nitrogen to give pyridine ''N''-oxide. The oxidation can be achieved with peracids:
:C5H5N + RCO3H → C5H5NO + RCO2H
Some electrophilic substitutions on the pyridine are usefully effected using pyridine ''N''-oxide followed by deoxygenation. Addition of oxygen suppresses further reactions at nitrogen atom and promotes substitution at the 2- and 4-carbons. The oxygen atom can then be removed, e.g. using zinc dust.
Oxidation of pyridine occurs at nitrogen to give pyridine ''N''-oxide. The oxidation can be achieved with peracids:
:C5H5N + RCO3H → C5H5NO + RCO2H
Some electrophilic substitutions on the pyridine are usefully effected using pyridine ''N''-oxide followed by deoxygenation. Addition of oxygen suppresses further reactions at nitrogen atom and promotes substitution at the 2- and 4-carbons. The oxygen atom can then be removed, e.g. using zinc dust.
Nucleophilic substitutions
In contrast to benzene ring, pyridine efficiently supports several nucleophilic substitutions. The reason for this is relatively lower electron density of the carbon atoms of the ring. These reactions include substitutions with elimination of a hydride ion and elimination-additions with formation of an intermediate aryne configuration, and usually proceed at the 2- or 4-position.Radical reactions
Pyridine supports a series of radical reactions, which is used in its Dimer (chemistry), dimerization to bipyridines. Radical dimerization of pyridine with elementalsodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable iso ...
or Raney nickel selectively yields 4,4'-bipyridine, or 2,2'-bipyridine, which are important precursor reagents in the chemical industry. One of the name reactions involving free radicals is the Minisci reaction. It can produce 2-''tert''-butylpyridine upon reacting pyridine with pivalic acid, silver nitrate and ammonium in sulfuric acid with a yield of 97%.#Joule, Joule, pp. 125–141
Reactions on the nitrogen atom
Hydrogenation and reduction
 Piperidine is produced by hydrogenation of pyridine with a nickel-, cobalt-, or ruthenium-based catalyst at elevated temperatures. The hydrogenation of pyridine to piperidine releases 193.8 kJ·mol−1, which is slightly less than the energy of the hydrogenation of
Piperidine is produced by hydrogenation of pyridine with a nickel-, cobalt-, or ruthenium-based catalyst at elevated temperatures. The hydrogenation of pyridine to piperidine releases 193.8 kJ·mol−1, which is slightly less than the energy of the hydrogenation of benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
(205.3 kJ·mol−1).
Partially hydrogenated derivatives are obtained under milder conditions. For example, reduction with lithium aluminium hydride yields a mixture of 1,4-dihydropyridine, 1,2-dihydropyridine, and 2,5-dihydropyridine. Selective synthesis of 1,4-dihydropyridine is achieved in the presence of organometallic complexes of magnesium and zinc, and (Δ3,4)-tetrahydropyridine is obtained by electrochemical reduction of pyridine.
Lewis basicity and coordination compounds
Pyridine is a Lewis base, donating its pair of electrons to a Lewis acid. Its Lewis base properties are discussed in the ECW model. Its relative donor strength toward a series of acids, versus other Lewis bases, can be illustrated by ECW model, C-B plots. One example is the sulfur trioxide pyridine complex (melting point 175 °C), which is a sulfation agent used to convert alcohols to sulfate esters. Pyridine-borane (, melting point 10–11 °C) is a mild reducing agent.Applications
Pesticides and pharmaceuticals
The main use of pyridine is as a precursor to the herbicides paraquat and diquat. The first synthesis step of insecticide chlorpyrifos consists of the chlorination of pyridine. Pyridine is also the starting compound for the preparation of pyrithione-based fungicides. Cetylpyridinium chloride, Cetylpyridinium and laurylpyridinium, which can be produced from pyridine with a Zincke reaction, are used as antiseptic in oral and dental care products. Pyridine is easily attacked by alkylating agents to give ''N''-alkylpyridinium salts. One example is cetylpyridinium chloride. It is also used in the textile industry to improve network capacity of cotton.
It is also used in the textile industry to improve network capacity of cotton.
Laboratory use
Pyridine is used as a polar, basic, low-reactive solvent, for example in Knoevenagel condensations. It is especially suitable for the dehalogenation, where it acts as the base for the elimination reaction. In esterifications and acylations, pyridine activates the carboxylic acid chlorides and anhydrides. Even more active in these reactions are the derivatives 4-dimethylaminopyridine (DMAP) and 4-(1-pyrrolidinyl) pyridine. Pyridine is also used as a base in somecondensation reaction
In organic chemistry, a condensation reaction is a type of chemical reaction in which two molecules are combined to form a single molecule, usually with the loss of a small molecule such as water. If water is lost, the reaction is also known as a ...
s.
Reagents
Hazards
Pyridine is a toxic, flammable liquid with a strong and unpleasant fishy odour. Its odour threshold of 0.04 to 20 ppm is close to its threshold limit value, threshold limit of 5 ppm for adverse effects, thus most (but not all) adults will be able to tell when it is present at harmful levels. Pyridine easily dissolves in water and harms both animals and plants in aquatic systems.Fire
Pyridine has a flash point of 17 °C and is therefore highly flammable. Combustion produces toxic fumes which can includebipyridine Bipyridines also known as bipyridyls, dipyridyls, and dipyridines, are a family of chemical compounds with the formula (C5H4N)2, consisting of two pyridyl (C5H4N) rings. Pyridine is an aromatic nitrogen-containing heterocycle. Bipyridines are of sig ...
s, nitrogen oxides, and carbon monoxide.
Short-term exposure
Pyridine can cause chemical burns on contact with the skin and its fumes may be irritating to the eyes or upon inhalation. Pyridine depresses the nervous system giving symptoms similar to intoxication with vapor concentrations of above 3600 parts per million, ppm pose a greater health risk. The effects may have a delayed onset of several hours and include dizziness, headache, ataxia, lack of coordination, nausea, salivation, and loss of appetite. They may progress into abdominal pain, pulmonary congestion and unconsciousness. The lowest known lethal dose (LDLo) for the ingestion of pyridine in humans is 500 mg·kg−1.Long-term exposure
Prolonged exposure to pyridine may result in liver, heart and kidney damage. Evaluations as a possible carcinogenic agent showed that there is inadequate evidence in humans for the carcinogenicity of pyridine, although there is sufficient evidence in experimental animals. Therefore, International Agency for Research on Cancer, IARC considers pyridine as possibly carcinogenic to humans (Group 2B).Occurrence
Trace amounts of up to 16 µg·m−3 have been detected in tobacco smoke. Minor amounts of pyridine are released into environment from some industrial processes such as steel manufacture, processing of oil shale, coal gasification, coking plants and Incineration, incinerators. The atmosphere at oil shale processing plants can contain pyridine concentrations of up to 13 µg·m−3, and 53 µg·m−3 levels were measured in the groundwater in the vicinity of a coal gasification plant. According to a study by the US National Institute for Occupational Safety and Health, about 43,000 Americans work in contact with pyridine.In foods
Pyridine has historically been added to foods to give them a bitter flavour, although this practise is now banned in the U.S. It may still be added toethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl ...
to make it unsuitable for drinking.
Metabolism
 Exposure to pyridine would normally lead to its inhalation and absorption in the lungs and gastrointestinal tract, where it either remains unchanged or is metabolism, metabolized. The major products of pyridine metabolism are ''N''-methylpyridiniumhydroxide, which are formed by N-methyltransferase, ''N''-methyltransferases (e.g., pyridine N-methyltransferase, pyridine ''N''-methyltransferase), as well as pyridine ''N''-oxide, and 2-, 3-, and 4-hydroxypyridine, which are generated by the action of monooxygenase. In humans, pyridine is metabolized only into ''N''-methylpyridiniumhydroxide.
Exposure to pyridine would normally lead to its inhalation and absorption in the lungs and gastrointestinal tract, where it either remains unchanged or is metabolism, metabolized. The major products of pyridine metabolism are ''N''-methylpyridiniumhydroxide, which are formed by N-methyltransferase, ''N''-methyltransferases (e.g., pyridine N-methyltransferase, pyridine ''N''-methyltransferase), as well as pyridine ''N''-oxide, and 2-, 3-, and 4-hydroxypyridine, which are generated by the action of monooxygenase. In humans, pyridine is metabolized only into ''N''-methylpyridiniumhydroxide.
Environmental fate
Pyridine is readily degraded by bacteria to ammonia and carbon dioxide. The unsubstituted pyridine ring degrades more rapidly than picoline, lutidine, chloropyridine, or aminopyridines, and a number of pyridine degraders have been shown to overproduce riboflavin in the presence of pyridine. Ionizable ''N''-heterocyclic compounds, including pyridine, interact with environmental surfaces (such as soils and sediments) via multiple pH-dependent mechanisms, including partitioning to soil organic matter, cation exchange, and surface complexation. Such adsorption to surfaces reduces bioavailability of pyridines for microbial degraders and other organisms, thus slowing degradation rates and reducing ecotoxicity.Nomenclature
The systematic name of pyridine, within the Hantzsch–Widman nomenclature recommended by the IUPAC, is '. However, systematic names for simple compounds are used very rarely; instead, heterocyclic nomenclature follows historically established common names. IUPAC discourages the use of in favor of ''pyridine''. The numbering of the ring atoms in pyridine starts at the nitrogen (see infobox). An allocation of positions by letter of the Greek alphabet (α-γ) and the Arene substitution patterns, substitution pattern nomenclature common for homoaromatic systems (''ortho'', ''meta'', ''para'') are used sometimes. Here α (''ortho''), β (''meta''), and γ (''para'') refer to the 2, 3, and 4 position, respectively. The systematic name for the pyridine derivatives is ''pyridinyl'', wherein the position of the substituted atom is preceded by a number. However, the historical name ''pyridyl'' is encouraged by the IUPAC and used instead of the systematic name. The cationic derivative formed by the addition of an electrophile to the nitrogen atom is called ''pyridinium
Pyridinium refers to the cation . It is the conjugate acid of pyridine. Many related cations are known involving substituted pyridines, e.g. picolines, lutidines, collidines. They are prepared by treating pyridine with acids.
As pyridine is oft ...
''.
See also
* 6-membered aromatic rings with one carbon replaced by another group: borabenzene, silabenzene, germabenzene, stannabenzene, pyridine, phosphorine, arsabenzene, stibabenzene, bismabenzene, pyrylium, thiopyrylium, selenopyrylium, telluropyrylium * 6-membered rings with two nitrogen atoms:diazine
In organic chemistry, diazines are a group of organic compounds having the molecular formula . Each contains a benzene ring in which two of the C-H fragments have been replaced by isolobal nitrogen. There are three structural isomers:
* pyridaz ...
s
* 6-membered rings with three nitrogen atoms: triazines
* 6-membered rings with four nitrogen atoms: tetrazines
* 6-membered rings with five nitrogen atoms: pentazine
* 6-membered rings with six nitrogen atoms: hexazine
References
Bibliography
* *External links
Synthesis and properties of pyridines
at chemsynthesis.com
{{Authority control Pyridines, Amine solvents Foul-smelling chemicals Aromatic bases Simple aromatic rings Functional groups Aromatic solvents