Prescription Bottle on:
[Wikipedia]
[Google]
[Amazon]
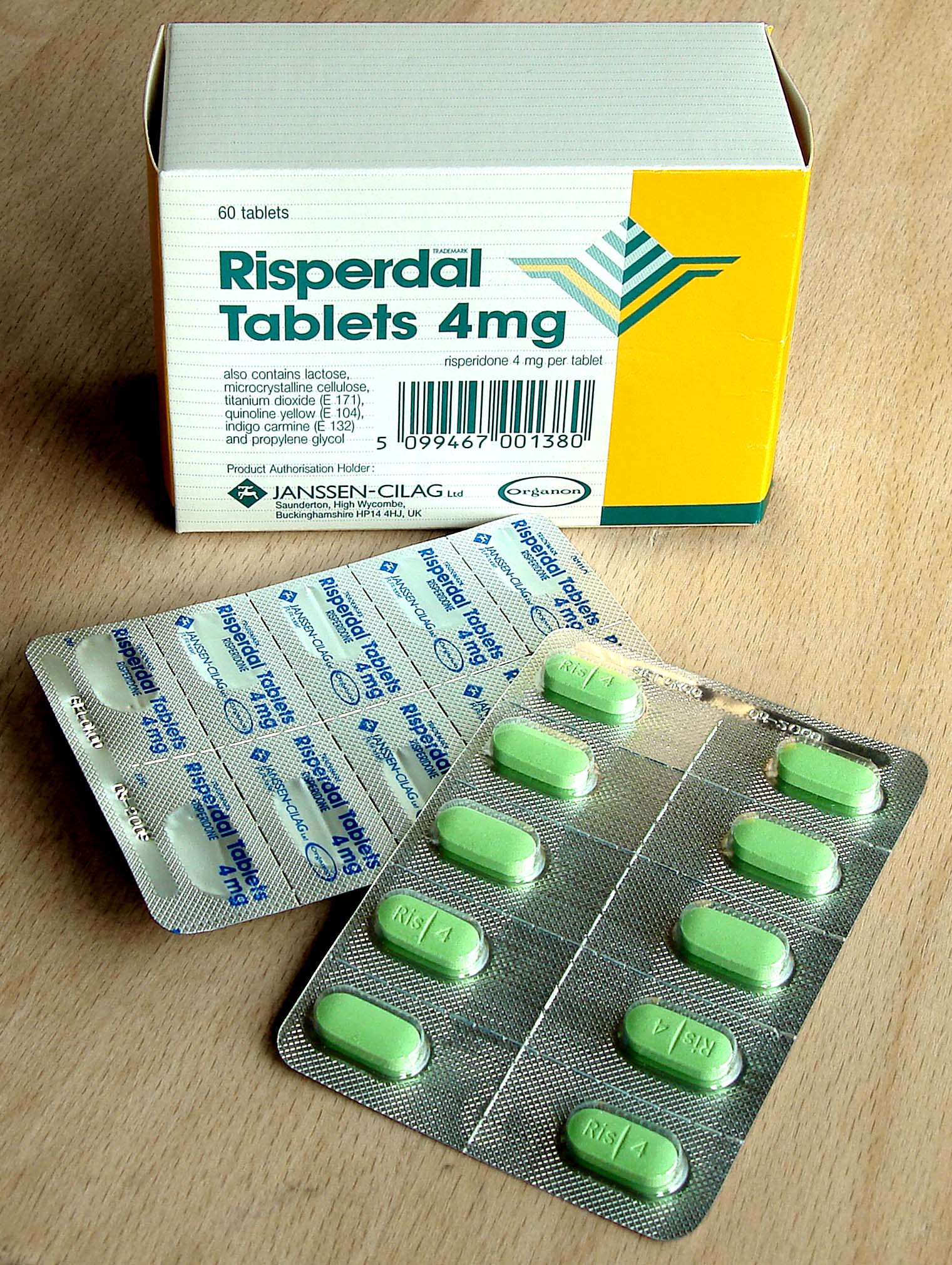 Pharmaceutical packaging (or drug packaging) is the packages and the
Pharmaceutical packaging (or drug packaging) is the packages and the
 Bulk pharmaceuticals can be shipped to another pharmaceutical company for further processing, to a
Bulk pharmaceuticals can be shipped to another pharmaceutical company for further processing, to a  Drugs under prescription control are sent to pharmacies in
Drugs under prescription control are sent to pharmacies in
 Formed solid
Formed solid
 Many pharmaceutical products are sensitive to heat or cold. Controlled distribution systems and sometimes
Many pharmaceutical products are sensitive to heat or cold. Controlled distribution systems and sometimes
 All aspects of pharmaceutical production, including packaging, are tightly controlled and have regulatory requirements. Uniformity, cleanliness (
All aspects of pharmaceutical production, including packaging, are tightly controlled and have regulatory requirements. Uniformity, cleanliness (
File:Childproof.jpg, Example of two types of child-resistant safety caps
File:Animal medication veterinary pharmaceutical bottles.JPG, Veterinary supplies
File:Pill box with pills.JPG, Pill box for home
File:Components of an Adalimumab-Humira pen - annotated.jpg, Components of injectable package
BECONASE nasal spray.JPG, Nasal spray
File:COVID-19 Vaccine vial and syringe - US Census.jpg, Vial of vaccine and syringe
Image:Infuuszakjes.jpg, Intravenous therapy
File:Fucidin 2pct 15g.jpg, Tube of ointment
File:Ampoule pharmaceutique.JPG,
* anon, Guidance for Industry: Container Closure Systems for Packaging Human Drugs and Biologics, May 1999, Food and Drug Administration, Center for Drug Evaluation and Research,
* Lockhart, H., and Paine, F.A., "Packaging of Pharmaceuticals and Healthcare Products", 2006, Blackie, * Pilchik, R., "Validating Medical Packaging" 2002, * Rosette, J. L, "Improving Tamper-Evident Packaging: Problems, Tests and Solutions", 1992 * Soroka, W, "Fundamentals of Packaging Technology", IoPP, 2002, *Soroka, W, Illustrated Glossary of Packaging Terminology, Institute of Packaging Professionals
* Yam, K. L., "Encyclopedia of Packaging Technology", John Wiley & Sons, 2009,
Processing Radioactive Materials
- large set of images by the
FDA Compliance Policy Guides - CPG Sec. 450.500 Tamper-Resistant Packaging Requirements for Certain Over-the-Counter Human Drug Products
{{packaging Medicine storage containers Packaging Labels Pharmaceutical industry Drug delivery devices
 Pharmaceutical packaging (or drug packaging) is the packages and the
Pharmaceutical packaging (or drug packaging) is the packages and the packaging
Packaging is the science, art and technology of enclosing or protecting products for distribution, storage, sale, and use. Packaging also refers to the process of designing, evaluating, and producing packages. Packaging can be described as a co ...
processes for pharmaceutical preparations. It involves all of the operations from production through drug distribution
The distribution of medications has special drug safety and security considerations. Some drugs require cold chain management in their distribution.
The industry uses track and trace technology, though the timings for implementation and the i ...
channels to the end consumer.
Pharmaceutical packaging is highly regulated but with some variation in the details, depending on the country of origin or the region. Several common factors can include: assurance of patient safety, assurance of the efficacy of the drug through the intended shelf life
Shelf life is the length of time that a commodity may be stored without becoming unfit for use, consumption, or sale. In other words, it might refer to whether a commodity should no longer be on a pantry shelf (unfit for use), or no longer on a ...
, uniformity of the drug through different production lots, thorough documentation of all materials and processes, control of possible migration of packaging components into the drug, control of degradation of the drug by oxygen, moisture, heat, etc., prevention of microbial
A microorganism, or microbe,, ''mikros'', "small") and ''organism'' from the el, ὀργανισμός, ''organismós'', "organism"). It is usually written as a single word but is sometimes hyphenated (''micro-organism''), especially in olde ...
contamination, sterility, etc. Packaging is often involved in dispensing, dosing, and use of the pharmaceutical product. Communication of proper use and cautionary labels are also regulated. Packaging is an integral part of pharmaceutical product.
Segments of usage
Pharmaceutical packaging can often be thought of by the segment in the distribution system being encountered and by the functions needed by the user of the package. Packaging requirements are different. Bulk pharmaceuticals can be shipped to another pharmaceutical company for further processing, to a
Bulk pharmaceuticals can be shipped to another pharmaceutical company for further processing, to a contract packager
A contract packager, or copacker, is a company that packages products for their clients. The packaging and labeling services can be used for many types of products including foods, pharmaceuticals, household products, and industrial products.
Fu ...
for forming unit packs, to international customers, etc. Bulk shipments might be in fiber drums (with plastic liners), bulk box
A bulk box, also known as a bulk bin, skid box, pallet box, bin box, gaylord, or octabin is a pallet-size box used for storage and shipping of bulk quantities.
In the U.S. and Canada, the term ''gaylord'' is sometimes used for triplewall corru ...
es, corrugated box
Corrugated box design is the process of matching design factors for corrugated fiberboard boxes with the functional physical, processing and end-use requirements. Packaging engineers work to meet the performance requirements of a box while control ...
es with liners, intermediate bulk container
Intermediate bulk containers (also known as IBC tank, IBC tote, IBC, or pallet tank) are industrial-grade containers engineered for the mass handling, transport, and storage of liquids, semi-solids, pastes, or solids. The two main categories of I ...
s, and other shipping container
A shipping container is a container with strength suitable to withstand shipment, storage, and handling. Shipping containers range from large reusable steel boxes used for intermodal shipments to the ubiquitous corrugated boxes. In the context of ...
s.
Smaller bulk packs can be shipped to pharmacies, particularly compounding pharmacies. The liquids or powders can be measured and put into primary packages.
Shipments to medical professionals could be at hospitals, nursing homes, veterinarians, dentists, etc. These packaged pharmaceuticals are intended to be dispensed and administered by professionally trained and certified personnel.
multi-pack
A multi-pack also known as multipack is packaging that combines or holds multiple items or smaller packages.
Functions
Multi-packs can be used to:
* Combine several items for a larger unit of sale, often with a reduced individual cost
* Provide ...
s of unit packs or in bottles containing many hundreds of capsules. Typically a pharmacist prepares the final form of the unit pack or places a lower count of capsules in a small bottle for the customer. In a pharmacy, pharmacists are available to answer questions and to ensure that proper documentation is provided. Internet pharmacies mail the prescribed drugs to the customer; boxes or mailing envelopes are used. Child resistant packaging is often required on the unit packs; if requested, a pharmacist is allowed put drugs in a bottle with easy open features.
Over-the-counter drugs are sold in drug stores, grocery stores, and diverse retail outlets. Usually the package needs to have all the usage information available. Packages often need to have tamper resistant
Tamperproofing, conceptually, is a methodology used to hinder, deter or detect unauthorised access to a device or circumvention of a security system. Since any device or system can be foiled by a person with sufficient knowledge, equipment, and ti ...
features and child-resistant packaging
Child-resistant packaging or CR packaging is special packaging used to reduce the risk of children ingesting hazardous materials. This is often accomplished by the use of a special safety cap. It is required by regulation for prescription drugs, o ...
.
Usually the packaging and labeling of dietary supplement
A dietary supplement is a manufactured product intended to supplement one's diet by taking a pill, capsule, tablet, powder, or liquid. A supplement can provide nutrients either extracted from food sources or that are synthetic in order ...
s, homeopathic
Homeopathy or homoeopathy is a pseudoscientific system of alternative medicine. It was conceived in 1796 by the German physician Samuel Hahnemann. Its practitioners, called homeopaths, believe that a substance that causes symptoms of a dise ...
drugs, and folk medicine
Traditional medicine (also known as indigenous medicine or folk medicine) comprises medical aspects of traditional knowledge that developed over generations within the folk beliefs of various societies, including indigenous peoples, before the ...
s are not regulated. Some producers voluntarily follow the regulations for over-the-counter drugs or regional Pharmacopoeia
A pharmacopoeia, pharmacopeia, or pharmacopoea (from the obsolete typography ''pharmacopœia'', meaning "drug-making"), in its modern technical sense, is a book containing directions for the identification of compound medicines, and published by ...
s.
Package forms
The wide variety of pharmaceutical solids, liquids, and gasses are packaged in a wide variety of packages. Some of the common primary packages are:Blister packs
 Formed solid
Formed solid unit dose
Dosage forms (also called unit doses) are pharmaceutical drug products in the form in which they are marketed for use, with a specific mixture of active ingredients and inactive components (excipients), in a particular configuration (such as a cap ...
s of pharmaceuticals ( capsules, suppositories
A suppository is a dosage form used to deliver medications by insertion into a body orifice where it dissolves or melts to exert local or systemic effects. There are three types of suppositories, each to insert into a different sections: rectal su ...
, tablets, etc.) are commonly packed in blister packs. In Europe about 85% of solid unit doses are packed in blister packs with only about 20% in North America.
Blister packs are pre-formed plastic/paper/foil packaging used for formed solid drugs. The primary component of a blister pack is a cavity or pocket made from a thermoformed plastic. This usually has a backing of paperboard
Paperboard is a thick paper-based material. While there is no rigid differentiation between paper and paperboard, paperboard is generally thicker (usually over 0.30 mm, 0.012 in, or 12 Inch#equivalences, points) than paper and has certain ...
or a lidding seal of aluminum foil
Aluminium foil (or aluminum foil in North American English; often informally called tin foil) is aluminium prepared in thin metal leaves with a thickness less than ; thinner gauges down to are also commonly used. Standard household foil is typ ...
or plastic film
Plastic film is a thin continuous polymeric material. Thicker plastic material is often called a "sheet". These thin plastic membranes are used to separate areas or volumes, to hold items, to act as barriers, or as printable surfaces.
Plas ...
. Blister packs are useful for protecting drugs against external factors, such as humidity and contamination for extended periods of time.
Blister packing machinery is readily available and is suited to validation processes.
Bottles
Bottles are commonly used for liquid pharmaceuticals as well as formed tablets and capsules. Glass is most common for liquids because it is inert and has excellent barrier properties. Various types of plastic bottles are used both by drug producers as well as by pharmacists in a pharmacy. Prescription bottles have been around since the 19th century. Throughout the 19th and 20th centuries, prescription medication bottles were called ''medicinal bottles''. There are many styles and shapes of prescription bottles. Bottles would often include cotton to cushion powdery, breakable pills. In modern times, pills are coated, and thus the inclusion of a cotton ball is no longer necessary. The U.S.National Institute of Health
The National Institutes of Health, commonly referred to as NIH (with each letter pronounced individually), is the primary agency of the United States government responsible for biomedical and public health research. It was founded in the late 1 ...
recommends consumers remove any cotton balls from opened pill bottles, as cotton balls may attract moisture into the bottle.
Prescription bottles come in several different colors, the most common of which being orange or light brown due to its ability to prevent ultraviolet light
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiation i ...
from degrading the potentially photosensitive contents through photochemical reactions Organic photochemistry encompasses organic reactions that are induced by the action of light. The absorption of ultraviolet light by organic molecules often leads to reactions. In the earliest days, sunlight was employed, while in more modern times ...
, while still letting enough visible light through for the contents to be easily visible. Other common colors include: Clear (for compounds that don't degrade in light), blue, dark brown, green, and various opaque hues.
Temperature
 Many pharmaceutical products are sensitive to heat or cold. Controlled distribution systems and sometimes
Many pharmaceutical products are sensitive to heat or cold. Controlled distribution systems and sometimes cold chain
A cold chain is a low temperature-controlled supply chain network. An unbroken cold chain is an uninterrupted series of refrigerated production, storage and distribution activities, along with associated equipment and logistics, which maintain qu ...
s are required.
A mail order or online pharmacy
An online pharmacy, internet pharmacy, or mail-order pharmacy is a pharmacy that operates over the Internet and sends orders to customers through mail, shipping companies, or online pharmacy web portal.
Online pharmacies include:
* Pharmacy ben ...
usually ships orders by mail services or by small parcel carrier. The shipment is not temperature-controlled and it may sit in a mail box upon delivery. Conditions can include high or low temperatures outside of the recommended storage conditions for certain products. For example, the USFDA
The United States Food and Drug Administration (FDA or US FDA) is a federal agency of the Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food s ...
found that the temperature in a steel mailbox painted black could reach 136 °F (58 °C) in full sun while the ambient air temperature was 101 °F (38 °C). Insulated mailing envelopes are sometimes used.
Larger shipments are sent in insulated shipping container Insulated shipping containers are a type of packaging used to ship temperature sensitive products such as foods, pharmaceuticals, organs, blood, biologic materials, vaccines and chemicals. They are used as part of a cold chain to help maintain pro ...
s with dry ice
Dry ice is the solid form of carbon dioxide. It is commonly used for temporary refrigeration as CO2 does not have a liquid state at normal atmospheric pressure and sublimates directly from the solid state to the gas state. It is used primarily a ...
or gel pack
An ice pack or gel pack is a portable bag filled with water, refrigerant gel, or liquid, meant to provide cooling. They can be divided into the reusable type, which works as a thermal mass and requires freezing, or the instant type, which cools ...
s. A digital temperature data logger A temperature data logger, also called temperature monitor, is a portable measurement instrument that is capable of autonomously recording temperature over a defined period of time. The digital data can be retrieved, viewed and evaluated after it h ...
or a time temperature indicator
A time temperature indicator (TTI) is a device or smart label that shows the accumulated time-temperature history of a product. Time temperature indicators are commonly used on food, pharmaceutical, and medical products to indicate exposure to ex ...
is often enclosed to monitor the temperature inside the container for its entire shipment.
Moisture
Many dry pharmaceuticals are sensitive to moisture. Tablets may become unstable and the drug may degrade. High barrier packaging (including seals) is necessary but, by itself, is often not enough.Shelf life
Shelf life is the length of time that a commodity may be stored without becoming unfit for use, consumption, or sale. In other words, it might refer to whether a commodity should no longer be on a pantry shelf (unfit for use), or no longer on a ...
of a moisture-sensitive drug can be extended by means of desiccants.
Several types of dessicants are available; the type and quantity need to be matched to the particular drug and package. One common method is to include a small packet of dessicant in a bottle. Other methods of including desiccants attached to the inner surface or in the material have recently been developed.
Counterfeiting
Counterfeit drugs are a serious problem. People can potentially ingest useless or dangerous drugs without their knowledge. Custom package seals,authentication
Authentication (from ''authentikos'', "real, genuine", from αὐθέντης ''authentes'', "author") is the act of proving an assertion, such as the identity of a computer system user. In contrast with identification, the act of indicati ...
labels, hologram
Holography is a technique that enables a wavefront to be recorded and later re-constructed. Holography is best known as a method of generating real three-dimensional images, but it also has a wide range of other Holography#Applications, applic ...
s, and security printing
Security printing is the field of the printing industry that deals with the printing of items such as banknotes, cheques, passports, tamper-evident labels, security tapes, product authentication, stock certificates, postage stamps and identity ca ...
can be valued parts of an entire security system. They help verify that enclosed drugs are what the package says they are. Drug counterfeiters, however, often work with package counterfeiters, some of whom can be sophisticated. No packaging system is completely secure.
Prescription labels
Medication packaging includes a document that provides information about that drug and its use. In the US, this information is overseen by the Center for Drug Research and Evaluation (CDER), a branch of the Food and Drug Administration (FDA). Forprescription medication
A prescription drug (also prescription medication or prescription medicine) is a pharmaceutical drug that legally requires a medical prescription to be dispensed. In contrast, over-the-counter drugs can be obtained without a prescription. The rea ...
s, the insert is technical
Technical may refer to:
* Technical (vehicle), an improvised fighting vehicle
* Technical analysis, a discipline for forecasting the future direction of prices through the study of past market data
* Technical drawing, showing how something is co ...
, and provides information for medical professionals
A health professional, healthcare professional, or healthcare worker (sometimes abbreviated HCW) is a provider of health care treatment and advice based on formal training and experience. The field includes those who work as a nurse, physician (su ...
about how to prescribe the drug. Package inserts for prescription drugs often include a separate document called a "patient package insert" with information written in plain language
Plain language is writing designed to ensure the reader understands as quickly, easily, and completely as possible. Plain language strives to be easy to read, understand, and use. It avoids verbose, convoluted language and jargon. In many countri ...
intended for the end-user
In product development, an end user (sometimes end-user) is a person who ultimately uses or is intended to ultimately use a product. The end user stands in contrast to users who support or maintain the product, such as sysops, system administrat ...
-- the person who will take the drug or administer the drug to another person. Inserts for over-the-counter medication
Over-the-counter (OTC) drugs are medicines sold directly to a consumer without a requirement for a prescription from a healthcare professional, as opposed to prescription drugs, which may be supplied only to consumers possessing a valid prescr ...
s are also written plainly.
In the US the document is called "prescribing information" or the "package insert
A package insert is a document included in the package of a medication that provides information about that drug and its use. For prescription medications, the insert is technical, providing information for medical professionals about how to pre ...
" (PI) and layperson's document is called the "patient package insert" (PPI). In Europe the technical document is called the "summary of product characteristics" and the document for end-users is called the "package leaflet".
The bottle or box also has information printed on it, intended for the person taking the medication.
Packaging production
washdown
Washdown (also wash down) is the process of cleaning or washing a surface for appearance, sanitation, or removal of contamination. It may involve pressure washing. Sometimes wash down involves rinsing with fresh water; other times it involves use ...
), sterility, and other requirements are needed to maintain Good Manufacturing Practice
Current good manufacturing practices (cGMP) are those conforming to the guidelines recommended by relevant agencies. Those agencies control the authorization and licensing of the manufacture and sale of food and beverages, cosmetics, pharmaceutica ...
s.
Product safety management is vital. A complete Quality Management System
A quality management system (QMS) is a collection of business processes focused on consistently meeting customer requirements and enhancing their satisfaction. It is aligned with an organization's purpose and strategic direction (ISO 9001:2015). I ...
must be in place. Validation involves collecting documentary evidence of all aspects of compliance. Hazard analysis and critical control points
Hazard analysis and critical control points, or HACCP (), is a systematic preventive approach to food safety from biological, chemical, and physical hazards in production processes that can cause the finished product to be unsafe and designs mea ...
is a methodology which has been proven useful. Quality assurance extends beyond the packaging operations through distribution and cold chain management; Good distribution Distribution may refer to:
Mathematics
*Distribution (mathematics), generalized functions used to formulate solutions of partial differential equations
* Probability distribution, the probability of a particular value or value range of a vari ...
practice is often a regulatory requirement. Track and trace
In the distribution and logistics of many types of products, track and trace or tracking and tracing concerns a process of determining the current and past locations (and other information) of a unique item or property.
This concept can be s ...
systems are usually required.
With a large portion of pharmaceutical packaging being outsourced to contract packager
A contract packager, or copacker, is a company that packages products for their clients. The packaging and labeling services can be used for many types of products including foods, pharmaceuticals, household products, and industrial products.
Fu ...
s, additional demand is being placed on specialty areas, i.e. specialty dosage forms.
Examples
Ampoule
An ampoule (also ampul and ampule) is a small sealed vial which is used to contain and preserve a sample, usually a solid or liquid. Ampoules are usually made of glass.
Modern ampoules are most commonly used to contain pharmaceuticals and chem ...
s
Drop counter and vial.JPG, Drop counter
Tpn 3bag.jpg, Parenteral nutrition
Parenteral nutrition (PN) is the feeding of nutritional products to a person intravenously, bypassing the usual process of eating and digestion. The products are made by pharmaceutical compounding companies. The person receives a nutritional mix ...
kit
File:Enema prepared, disposable.jpg, A prepared, disposable enema
An enema, also known as a clyster, is an injection of fluid into the lower bowel by way of the rectum.Cullingworth, ''A Manual of Nursing, Medical and Surgical'':155 The word enema can also refer to the liquid injected, as well as to a device ...
File:DoD random drug test specimen bottle.png, Drug test bottle with authentication seal
Image:Inhaler2.jpg, A metered-dose inhaler
A metered-dose inhaler (MDI) is a device that delivers a specific amount of medication to the lungs, in the form of a short burst of aerosolized medicine that is usually self-administered by the patient via inhalation. It is the most commonly used ...
(MDI)
File:Epi-Pen 2016.jpg , Epinephrine autoinjector
File:Medicijnflesje met doorprikstop.JPG, Tearable metal band on pharmaceutical bottle
File:Faltschachteln mit integriertem RFID-Tag.jpg, RFID chip built into drug package
File:Walgreens Prescription Bottle.jpg, Prescription Bottle
File:Ögondroppar2.jpg, Sterile single-dose vial
A vial (also known as a phial or flacon) is a small glass or plastic vessel or bottle, often used to store medication as liquids, powders or capsules. They can also be used as scientific sample vessels; for instance, in autosampler devices i ...
of eye drops
File:Embalaje monodosis de medicamentos.jpg, Unit dose packet
Packet may refer to:
* A small container or pouch
** Packet (container), a small single use container
** Cigarette packet
** Sugar packet
* Network packet, a formatted unit of data carried by a packet-mode computer network
* Packet radio, a form ...
s with full identification (text and bar codes)
File:CDC 2019-nCoV Laboratory Test Kit.jpg, alt=CDC 2019-nCoV Laboratory Test Kit.jpg, US CDC's COVID-19 laboratory test kit
See also
*Child-resistant packaging
Child-resistant packaging or CR packaging is special packaging used to reduce the risk of children ingesting hazardous materials. This is often accomplished by the use of a special safety cap. It is required by regulation for prescription drugs, o ...
* Codex Alimentarius
The Codex Alimentarius () is a collection of internationally recognized standards, codes of practice, guidelines, and other recommendations published by the Food and Agriculture Organization of the United Nations relating to food, food production ...
*Track and trace
In the distribution and logistics of many types of products, track and trace or tracking and tracing concerns a process of determining the current and past locations (and other information) of a unique item or property.
This concept can be s ...
*Product recall
A product recall is a request from a manufacturer to return a product after the discovery of safety issues or product defects that might endanger the consumer or put the maker/seller at risk of legal action.
The recall is an effort to limit ruin ...
*Contract manufacturing organization
A contract manufacturing organization (CMO), more recently referred to (and more commonly used now) as a contract development and manufacturing organization (CDMO) to avoid the acronym confusion of Chief Medical Officer or Clinical Monitoring Organ ...
*European Pharmacopoeia
The ''European Pharmacopoeia'' (''Pharmacopoeia Europaea'', ''Ph. Eur.'') is a major regional pharmacopoeia which provides common quality standards throughout the pharmaceutical industry in Europe to control the quality of medicines, and the su ...
*ClearRx
ClearRx is a trademark for a design for prescription drug packaging, designed by design student Deborah Adler as a Thesis, thesis project and adopted by Target Corporation (with refinements by Industrial design, industrial designer Klaus Rosburg) f ...
References
General references
* anon, Guidance for Industry:Q8 (R2) Pharmaceutical Development, US FDA, 2009* anon, Guidance for Industry: Container Closure Systems for Packaging Human Drugs and Biologics, May 1999, Food and Drug Administration, Center for Drug Evaluation and Research,
* Lockhart, H., and Paine, F.A., "Packaging of Pharmaceuticals and Healthcare Products", 2006, Blackie, * Pilchik, R., "Validating Medical Packaging" 2002, * Rosette, J. L, "Improving Tamper-Evident Packaging: Problems, Tests and Solutions", 1992 * Soroka, W, "Fundamentals of Packaging Technology", IoPP, 2002, *Soroka, W, Illustrated Glossary of Packaging Terminology, Institute of Packaging Professionals
* Yam, K. L., "Encyclopedia of Packaging Technology", John Wiley & Sons, 2009,
External links
Processing Radioactive Materials
- large set of images by the
IAEA
The International Atomic Energy Agency (IAEA) is an intergovernmental organization that seeks to promote the peaceful use of nuclear energy and to inhibit its use for any military purpose, including nuclear weapons. It was established in 1957 ...
showing automated package labelling and tracking for shipment of hazardous radioactive pharmaceuticals.
FDA Compliance Policy Guides - CPG Sec. 450.500 Tamper-Resistant Packaging Requirements for Certain Over-the-Counter Human Drug Products
{{packaging Medicine storage containers Packaging Labels Pharmaceutical industry Drug delivery devices