Polypyridine Complex on:
[Wikipedia]
[Google]
[Amazon]
Polypyridine complexes are 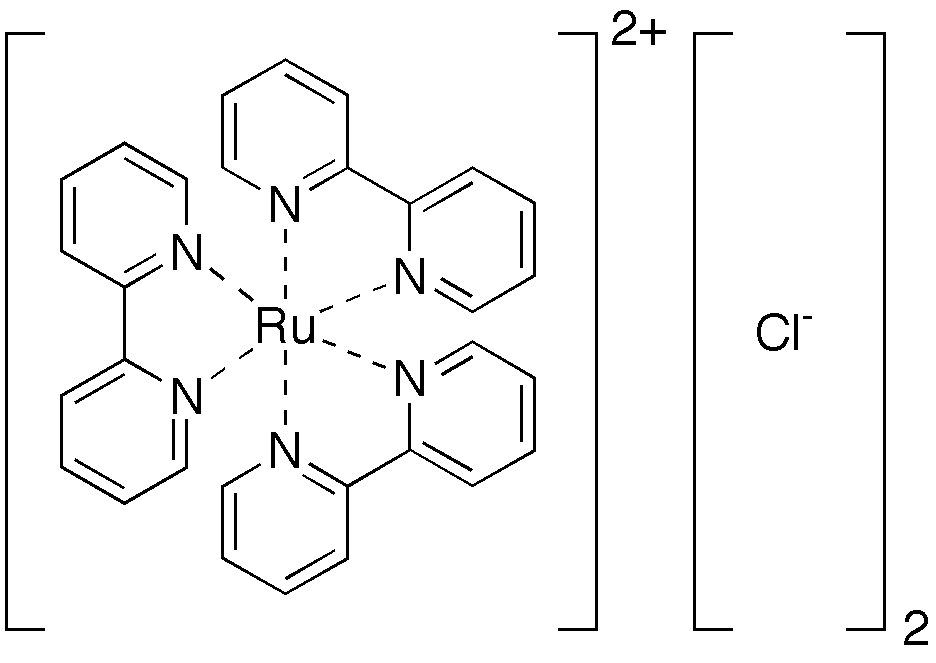 A well-known example of a polypyridine complex is the tris(bipyridine) derivative of ruthenium(II), u[(bpy)3sup>2+._This_complex_exhibits_intense_
A well-known example of a polypyridine complex is the tris(bipyridine) derivative of ruthenium(II), u[(bpy)3sup>2+._This_complex_exhibits_intense_
at room temperature, and its wavelength and lifetime can be tuned by substitution of either bipyridine or dithiolate moieties. Structural control is easier than for ruthenium complexes due to the square planar structure of the platinum complex.
Some other areas of investigation involves immobilizing these complexes on electrodes. Some polypyridyl complexes intercalate into DNA and show promise as drugs.Komor, Alexis C.; Barton, Jacqueline K. "The path for metal complexes to a DNA target" Chemical Communications 2013, vol. 49, 3617-3630.
coordination complex
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many ...
es containing polypyridine ligands, such as 2,2'-bipyridine
The comma is a punctuation mark that appears in several variants in different languages. It has the same shape as an apostrophe or single closing quotation mark () in many typefaces, but it differs from them in being placed on the baseline o ...
, 1,10-phenanthroline
1,10-Phenanthroline (phen) is a heterocyclic organic compound. It is a white solid that is soluble in organic solvents. The 1,10 refer to the location of the nitrogen atoms that replace CH's in the hydrocarbon called phenanthrene.
Abbreviated " ...
, or 2,2';6'2"-terpyridine
Terpyridine (2,2';6',2"-terpyridine, often abbreviated to Terpy or Tpy) is a heterocyclic compound derived from pyridine. It is a white solid that is soluble in most organic solvents. The compound is mainly used as a ligand in coordination chemist ...
.
Polypyridines are multidentate ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electr ...
s that confer characteristic properties to the metal complexes that they form. Some complexes strongly absorb light via a process called metal-to-ligand charge transfer (MLCT). The properties of these complexes can be tuned by changes in substituents. For example, electron donation, electron withdrawal, and π-conjugating groups, to the polypyridine moiety
Moiety may refer to:
Chemistry
* Moiety (chemistry), a part or functional group of a molecule
** Moiety conservation, conservation of a subgroup in a chemical species
Anthropology
* Moiety (kinship), either of two groups into which a society is ...
. The MLCT absorption band can be shifted, the emission wavelength can be changed, and the emission lifetime can be extended.
 A well-known example of a polypyridine complex is the tris(bipyridine) derivative of ruthenium(II), u[(bpy)3sup>2+._This_complex_exhibits_intense_
A well-known example of a polypyridine complex is the tris(bipyridine) derivative of ruthenium(II), u[(bpy)3sup>2+._This_complex_exhibits_intense_u[(bpy)3sup>2+._This_complex_exhibits_intense_luminescence">bpy)3.html"_;"title="u[(bpy)3">u[(bpy)3sup>2+._This_complex_exhibits_intense_luminescence_at_room_temperature_in_aqueous_solution._Another_example_is_a_platinum-bipyridine-dithiolate_complex,_Pt(bpy)(bdt),_in_which_bdt_denotes_a_1,2-benzenedithiolate_
. This complex also exhibits Fluorescence">photoluminescence
Photoluminescence (abbreviated as PL) is light emission from any form of matter after the absorption of photons (electromagnetic radiation). It is one of many forms of luminescence (light emission) and is initiated by photoexcitation (i.e. photon ...u[(bpy)3sup>2+._This_complex_exhibits_intense_luminescence">bpy)3.html"_;"title="u[(bpy)3">u[(bpy)3sup>2+._This_complex_exhibits_intense_luminescence_at_room_temperature_in_aqueous_solution._Another_example_is_a_platinum-bipyridine-dithiolate_complex,_Pt(bpy)(bdt),_in_which_bdt_denotes_a_1,2-benzenedithiolate_ion">anion_
An_ion_()_is_an_atom_or_molecule_with_a_net_electrical_charge.
The_charge_of_an_electron_is_considered_to_be_negative_by_convention_and_this_charge_is_equal_and_opposite_to_the_charge_of_a_proton,_which_is_considered_to_be_positive_by_convent_...
._This_complex_also_exhibits_Fluorescence.html" "title="ion.html" "title="luminescence.html" ;"title="bpy)3.html" ;"title="u[(bpy)3">u[(bpy)3sup>2+. This complex exhibits intense luminescence">bpy)3.html" ;"title="u[(bpy)3">u[(bpy)3sup>2+. This complex exhibits intense luminescence at room temperature in aqueous solution. Another example is a platinum-bipyridine-dithiolate complex, Pt(bpy)(bdt), in which bdt denotes a 1,2-benzenedithiolate ion">anion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...See also
*Transition metal complexes of 2,2'-bipyridine Transition metal complexes of 2,2'-bipyridine are coordination complexes containing one or more 2,2'-bipyridine ligands. Complexes have been described for all of the transition metals. Although few have any practical value, these complexes have be ...
References