Polyaspartate on:
[Wikipedia]
[Google]
[Amazon]
 Polyaspartic acid (PASA) is a
Polyaspartic acid (PASA) is a
 Many different routes lead to PASA. In the simplest and the oldest approach aspartic acid is heated to induce dehydration. In a subsequent step the resulting polysuccinimide is treated with aqueous
Many different routes lead to PASA. In the simplest and the oldest approach aspartic acid is heated to induce dehydration. In a subsequent step the resulting polysuccinimide is treated with aqueous
99–111
, isbn= 9780841231337 , chapter-url-access= registration , chapter-url= https://archive.org/details/hydrophilicpolym0000unse , url= https://archive.org/details/hydrophilicpolym0000unse/page/99 {{cite journal , first1= H. , last1= Pivcova , first2= V. , last2= Saudek , first3= J. , last3= Drobnik , first4= J. , last4= Vlasak , year= 1981 , title= NMR Study of Poly(aspartic acid). I. α-and β-Peptide Bonds in Poly(aspartic acid) Prepared by Thermal Polycondensation , journal= Biopolymers , volume= 20 , issue= 8 , pages=1605–1614 , doi= 10.1002/bip.1981.360200804 , s2cid= 85201969 {{cite journal , first1= Etso , last1= Kokufuta , first2= Shinnichiro , last2= Suzuki , first3= Kaoru , last3= Harad , year= 1978 , title= Temperature Effect on the Molecular Weight and the Optical Purity of Anhydropolyaspartic Acid Prepared by Thermal Polycondensation , journal= Bulletin of the Chemical Society of Japan , volume= 51 , issue= 5 , pages=1555–1556 , doi= 10.1246/bcsj.51.1555 , doi-access= free {{cite journal , first1= Takeshi , last1= Nakato , first2= Atsushi , last2= Kusuno , first3= Toyoji , last3= Kakuchi , year= 2000 , title= Synthesis of poly(succinimide) by bulk polycondensation of L-aspartic acid with an acid catalyst , journal= Journal of Polymer Science Part A: Polymer Chemistry , volume= 38 , issue= 1 , pages=117–122 , doi= 10.1002/(SICI)1099-0518(20000101)38:1<117::AID-POLA15>3.0.CO;2-F , bibcode= 2000JPoSA..38..117N , doi-access= free {{cite journal , first1= Yaquan , last1= Wang , first2= Yongjiang , last2= Hou , first3= Gang , last3= Ruan , first4= Ming , last4= Pan , first5= Tengfei , last5= Liu , year= 2003 , title= Study on the polymerization of aspartic acid catalyzed by phosphoric acid , journal= Journal of Macromolecular Science-Pure and Applied Chemistry , volume= A40 , issue= 3 , pages=293–307 , doi= 10.1081/MA-120018116 , s2cid= 85163135 {{cite journal , first1= Vanga S. , last1= Rao , first2= Philippe , last2= Lapointe , first3= Donald N. , last3= McGregor , year= 1993 , title= Temperature Effect on the Molecular Weight and the Optical Purity of Anhydropolyaspartic Acid Prepared by Thermal Polycondensation , journal= Makromolekulare Chemie-Macromolecular Chemistry and Physics , volume= 194 , issue= 4 , pages=1095–1104 , doi= 10.1002/macp.1993.021940405 {{cite journal , first1= Yasuyuki , last1= Soeda , first2= Kazunobu , last2= Toshima , first3= Shuichi , last3= Matsuma , year= 2003 , title= Sustainable enzymatic preparation of polyaspartate using a bacterial protease , journal= Biomacromolecules , volume= 4 , issue= 2 , pages=193–203 , doi= 10.1021/bm0200534 , pmid= 12625712 {{cite journal , first1= V. , last1= Saudek , first2= H. , last2= Pivcova , first3= J. , last3= Drobnik , year= 1981 , title= NMR Study of Poly(aspartic acid). II. α-and β-Peptide Bonds in Poly(aspartic acid) Prepared by Common Methods , journal= Biopolymers , volume= 20 , issue= 8 , pages=1615–1623 , doi= 10.1002/bip.1981.360200805 , s2cid= 84203387 {{cite patent , country = US , number = 5468838 , status = patent , title = Polysuccinimide, polyaspartic acid and their salts are prepared by reaction of maleic anhydride and ammonia, polycondensation of the resulting product in the presence of a solubilizing agent and, if appropriate, hydrolysis. , pubdate = 1995-11-21 , gdate = , fdate = 1994-03-14 , pridate = , invent1 = Boehmke, Gunter , invent2 = Schmitz, Gerd , assign1 = Bayer AG , assign2 = , class = {{Cite journal , last1 = Gross , first1 = Richard A. , last2 = Kalra , first2 = Bhanu , year = 2002 , title = Biodegradable Polymers for the Environment , journal = Science , volume = 297 , issue = 5582 , pages = 803–807 , doi = 10.1126/science.297.5582.803 , pmid = 12161646 , bibcode=2002Sci...297..803G, url = https://zenodo.org/record/1231185 {{Cite journal , first1= David , last1= Hasson , first2= Hilla , last2= Shemer , first3= Alexander , last3= Sher , year= 2011 , title= State of the Art of Friendly "Green" Scale Control Inhibitors: A Review Article , journal= Industrial & Engineering Chemistry Research , volume= 50 , issue= 12 , pages=7601–7607 , doi= 10.1021/ie200370v {{Cite journal , first1= M. J. , last1= Zahuriaan-Mehr , first2= A. , last2= Pourjavadi , first3= H. , last3= Salimi , first4= M. , last4= Kurdtabar , year= 2009 , title= Protein- and homo poly(amino acid)-based hydrogels with super-swelling properties , journal= Polymers for Advanced Technologies , volume= 20 , issue= 8 , pages=655–671 , doi= 10.1002/pat.1395 {{Cite journal , last1 = Thombre , first1 = Sunita M. , last2 = Sarwade , first2 = Bhimaro D. , year = 2005 , title = Synthesis and Biodegradability of Polyaspartic Acid: A Critical Review , journal = Journal of Macromolecular Science, Part A , volume = 42 , issue = 9 , pages = 1299–1315 , url = http://www.informaworld.com/index/718581646.pdf , doi = 10.1080/10601320500189604 , s2cid = 94818855 , 2 Polyamides Polyelectrolytes Chelating agents
 Polyaspartic acid (PASA) is a
Polyaspartic acid (PASA) is a biodegradable
Biodegradation is the breakdown of organic matter by microorganisms, such as bacteria and fungi. It is generally assumed to be a natural process, which differentiates it from composting. Composting is a human-driven process in which biodegradati ...
, water-soluble
In chemistry, solubility is the ability of a substance
Substance may refer to:
* Matter, anything that has mass and takes up space
Chemistry
* Chemical substance, a material with a definite chemical composition
* Drug substance
** Substan ...
condensation polymer
In polymer chemistry, condensation polymers are any kind of polymers whose process of polymerization involves a condensation reaction (i.e. a small molecule, such as water or methanol, is produced as a byproduct). Condensation polymers are for ...
based on the amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha ...
aspartic acid
Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α- amino acid that is used in the biosynthesis of proteins. Like all other amino acids, it contains an amino group and a carboxylic acid. Its α-amino group is in the pr ...
. It is a biodegradable replacement for water softeners and related applications. PASA can be chemically crosslinked with a wide variety of methods to yield PASA hydrogels
A gel is a semi-solid that can have properties ranging from soft and weak to hard and tough. Gels are defined as a substantially dilute cross-linked system, which exhibits no flow when in the steady-state, although the liquid phase may still dif ...
. The resulting hydrogels
A gel is a semi-solid that can have properties ranging from soft and weak to hard and tough. Gels are defined as a substantially dilute cross-linked system, which exhibits no flow when in the steady-state, although the liquid phase may still dif ...
are pH-sensitive such that under acidic conditions, they shrink, while the swelling capacity increases under alkaline conditions.
Sodium polyaspartate is a sodium salt of polyaspartic acid.
In nature, PASA has been found in as fragments of larger proteins
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respondi ...
with length up to 50 amino acids, but as of 2004 had not been isolated as a pure homo polymeric material from any natural source. The first isolation of synthetic oligomeric sodium polyaspartate
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable is ...
, obtained by thermal polycondensation
In polymer chemistry, condensation polymers are any kind of polymers whose process of polymerization involves a condensation reaction (i.e. a small molecule, such as water or methanol, is produced as a byproduct). Condensation polymers are for ...
of aspartic acid, was reported by Hugo Schiff
Hugo (Ugo) Schiff (26 April 1834 – 8 September 1915) was an Italian naturalized chemist. The son of a Jewish businessman and brother of the physiologist Moritz Schiff was German by nationality. He discovered Schiff bases and other imines, ...
in late 19th century. Later it was proposed that thermal polymerization process leads through polysuccinimide
Polysuccinimide (PSI), also known as polyanhydroaspartic acid or polyaspartimide, is formed during the thermal polycondensation of aspartic acid and is the simplest polyimide. Polysuccinimide is insoluble in water, but soluble in some aprotic dip ...
intermediate. Polyaspartic acid is produced industrially in both the acid form and as the sodium salt.
Properties and structure
Due to presence ofcarboxyl
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxyl ...
ic groups it is polyelectrolyte
Polyelectrolytes are polymers whose repeating units bear an electrolyte group. Polycations and polyanions are polyelectrolytes. These groups dissociate in aqueous solutions (water), making the polymers charged. Polyelectrolyte properties are th ...
with anion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
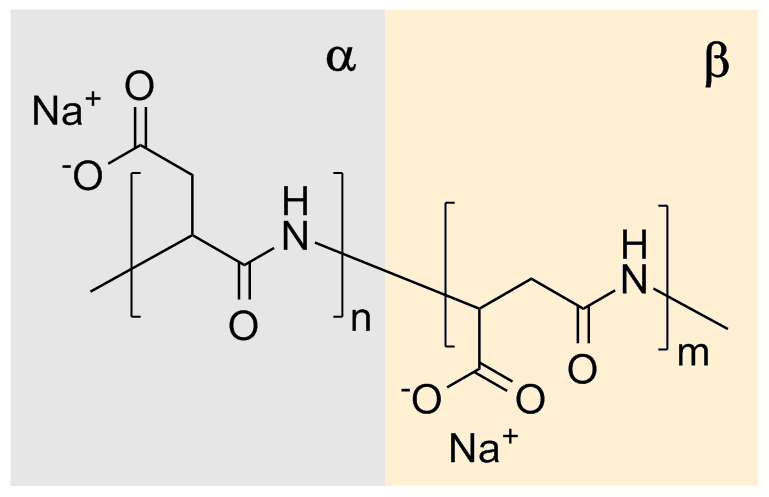
ic character. Naturally occurring PASA fragments consists of α,-linked L-aspartatic acid. In contrast, the repeating unit of synthetic polyaspartic acid may exist in four isomeric forms, depending on the stereochemistry of starting material (D- and L-aspartic acid
Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α- amino acid that is used in the biosynthesis of proteins. Like all other amino acids, it contains an amino group and a carboxylic acid. Its α-amino group is in the pr ...
) and synthetic procedure leading to α and β links. Due to the protein-like backbone (presence of amide bond in the backbone), PASA has suitable biodegradability
Biodegradation is the breakdown of organic matter by microorganisms, such as bacteria and fungi. It is generally assumed to be a natural process, which differentiates it from composting. Composting is a human-driven process in which biodegradati ...
.
Synthesis
 Many different routes lead to PASA. In the simplest and the oldest approach aspartic acid is heated to induce dehydration. In a subsequent step the resulting polysuccinimide is treated with aqueous
Many different routes lead to PASA. In the simplest and the oldest approach aspartic acid is heated to induce dehydration. In a subsequent step the resulting polysuccinimide is treated with aqueous sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and alkal ...
, which yields partial opening of the succinimide
Succinimide is an organic compound with the formula (CH2)2(CO)2NH. This white solid is used in a variety of organic syntheses, as well as in some industrial silver plating processes. The compound is classified as a cyclic imide. It may be prepared ...
rings. In this process sodium-DL-(α,β)-poly(aspartate) with 30% α-linkages and 70% β-linkages randomly distributed along the polymer chain, and racemized chiral center of aspartic acid is produced. There were many catalysts reported for improving thermal polymerization method. Main benefits from their application is increasing of the conversion rate and higher molecular weight of the product.
Polyaspartic acid can also be synthesized by polymerization of maleic anhydride
Maleic anhydride is an organic compound with the formula C2H2(CO)2O. It is the acid anhydride of maleic acid. It is a colorless or white solid with an acrid odor. It is produced industrially on a large scale for applications in coatings and po ...
in presence of ammonium hydroxide
Ammonia solution, also known as ammonia water, ammonium hydroxide, ammoniacal liquor, ammonia liquor, aqua ammonia, aqueous ammonia, or (inaccurately) ammonia, is a solution of ammonia in water. It can be denoted by the symbols NH3(aq). Although ...
.
High control over repeating unit isomers can be achieved by polymerization of N-carboxyanhydride (NCA) derivatives, by polymerization of aspartic acid esters or by application of enzyme catalyzed reaction. Pure homopolymers, D- or L-PASA with α- or β-links only, can be synthesized using those methods.
The polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many fo ...
reaction is an example of a step-growth polymerization
Step-growth polymerization refers to a type of polymerization mechanism in which bi-functional or multifunctional monomers react to form first dimers, then trimers, longer oligomers and eventually long chain polymers. Many naturally occurrin ...
to a polyamide
A polyamide is a polymer with repeating units linked by amide bonds.
Polyamides occur both naturally and artificially. Examples of naturally occurring polyamides are proteins, such as wool and silk. Artificially made polyamides can be made throug ...
. In one procedure, aspartic acid polymerizes at 180 °C
The degree Celsius is the unit of temperature on the Celsius scale (originally known as the centigrade scale outside Sweden), one of two temperature scales used in the International System of Units (SI), the other being the Kelvin scale. The d ...
concomitant with dehydration and the formation of a poly(succinimide
Succinimide is an organic compound with the formula (CH2)2(CO)2NH. This white solid is used in a variety of organic syntheses, as well as in some industrial silver plating processes. The compound is classified as a cyclic imide. It may be prepared ...
). The resulting polymer
A polymer (; Greek ''poly-'', "many" + '' -mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and ...
reacts with aqueous sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and alkal ...
, which hydrolyzes
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysis ...
one of the two amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is ...
bonds of the succinimide ring to form a sodium carboxylate. The remaining amide bond is thus the linkage between successive aspartate residues. Each aspartate residue is identified as α or β according to which carbonyl of it is part of the polymer chain. The α form has one carbon in the backbone in addition to the carbonyl itself (and a two-carbon sidechain) whereas the β form has two carbons in the backbone in addition to the carbonyl itself (and a one-carbon sidechain). This reaction gives a sodium poly(aspartate) composed of approximately 30% α-linkages and 70% β-linkages.
Applications
Polyaspartic acid and its derivatives are biodegradable alternatives to traditional polyanionic materials, in particularpolyacrylic acid
Poly(acrylic acid) (PAA; trade name Carbomer) is a polymer with the formula (CH2-CHCO2H)n. It is a derivative of acrylic acid (CH2=CHCO2H). In addition to the homopolymers, a variety of copolymers and crosslinked polymers, and partially deproto ...
. PASA has ability to inhibit deposition of calcium carbonate, calcium sulfate
Calcium sulfate (or calcium sulphate) is the inorganic compound with the formula CaSO4 and related hydrates. In the form of γ-anhydrite (the anhydrous form), it is used as a desiccant. One particular hydrate is better known as plaster of Paris, ...
, barium sulfate
Barium sulfate (or sulphate) is the inorganic compound with the chemical formula Ba SO4. It is a white crystalline solid that is odorless and insoluble in water. It occurs as the mineral barite, which is the main commercial source of barium ...
, and calcium phosphate and can be used as an antiscaling agent in cooling water systems, water desalination processes, and waste water treatment operations. In addition and due to its ability to chelate
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are ...
metal ions, it provides corrosion inhibition. It can also be used as biodegradable detergent and dispersant for various applications.
PASA also has a variety of biomedical
Biomedicine (also referred to as Western medicine, mainstream medicine or conventional medicine)
applications. Its high affinity with calcium has been exploited for targeting various forms of drug-containing carriers to the bone
A bone is a rigid organ that constitutes part of the skeleton in most vertebrate animals. Bones protect the various other organs of the body, produce red and white blood cells, store minerals, provide structure and support for the body, an ...
. The main component of bone
A bone is a rigid organ that constitutes part of the skeleton in most vertebrate animals. Bones protect the various other organs of the body, produce red and white blood cells, store minerals, provide structure and support for the body, an ...
is hydroxyapatite
Hydroxyapatite, also called hydroxylapatite (HA), is a naturally occurring mineral form of calcium apatite with the formula Ca5(PO4)3(OH), but it is usually written Ca10(PO4)6(OH)2 to denote that the crystal unit cell comprises two entities ...
(ca. 70%) (mineralized calcium phosphate
The term calcium phosphate refers to a family of materials and minerals containing calcium ions (Ca2+) together with inorganic phosphate anions. Some so-called calcium phosphates contain oxide and hydroxide as well. Calcium phosphates are wh ...
). Apart from bone targeting, PASA has been modified for other biomedical applications such as drug delivery
Drug delivery refers to approaches, formulations, manufacturing techniques, storage systems, and technologies involved in transporting a pharmaceutical compound to its target site to achieve a desired therapeutic effect. Principles related to dr ...
, surface coating, DNA delivery, mucoadhesion,
and beyond.
As it can be synthesized in an environmentally friendly way and is biodegradable
Biodegradation is the breakdown of organic matter by microorganisms, such as bacteria and fungi. It is generally assumed to be a natural process, which differentiates it from composting. Composting is a human-driven process in which biodegradati ...
, polyaspartate is a potential green
Green is the color between cyan and yellow on the visible spectrum. It is evoked by light which has a dominant wavelength of roughly 495570 Nanometre, nm. In subtractive color systems, used in painting and color printing, it is created by ...
alternative to several materials such as sodium polyacrylate
Sodium polyacrylate (ACR, ASAP, or PAAS), also known as waterlock, is a sodium salt of polyacrylic acid with the chemical formula ��CH2−CH(CO2Na)−sub>n and has broad applications in consumer products. This super-absorbent polymer (SAP) ha ...
used in disposable diaper
A diaper /ˈdaɪpə(r)/ ( American and Canadian English) or a nappy ( Australian English, British English, and Hiberno-English) is a type of underwear that allows the wearer to urinate or defecate without using a toilet, by absorbing or c ...
s and agriculture. It can act as a super-swelling material in diaper
A diaper /ˈdaɪpə(r)/ ( American and Canadian English) or a nappy ( Australian English, British English, and Hiberno-English) is a type of underwear that allows the wearer to urinate or defecate without using a toilet, by absorbing or c ...
s, feminine hygiene
Feminine hygiene products are personal care products used during menstruation, vaginal discharge, and other bodily functions related to the vulva and vagina. Products that are used during menstruation may also be called menstrual hygiene prod ...
products, and food packaging. The level of water uptake which is inversely related to the mechanical properties of the hydrogel can be tuned by changing the crosslinking density.
In addition and due to its water-solubility and ability to chelate
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are ...
metal ions, polyaspartate is a promising biodegradable anti-scaling
Scaling may refer to:
Science and technology
Mathematics and physics
* Scaling (geometry), a linear transformation that enlarges or diminishes objects
* Scale invariance, a feature of objects or laws that do not change if scales of length, energ ...
agent and a corrosion inhibitor
In chemistry, a corrosion inhibitor or anti-corrosive is a chemical compound that, when added to a liquid or gas, decreases the corrosion rate of a material, typically a metal or an alloy, that comes into contact with the fluid. The effectiveness ...
. Sodium polyaspartate also has a variety of biomedical
Biomedicine (also referred to as Western medicine, mainstream medicine or conventional medicine)
applications. Its high affinity with calcium has been exploited for targeting various forms of drug-containing carriers to the bone
A bone is a rigid organ that constitutes part of the skeleton in most vertebrate animals. Bones protect the various other organs of the body, produce red and white blood cells, store minerals, provide structure and support for the body, an ...
. The main component of bone
A bone is a rigid organ that constitutes part of the skeleton in most vertebrate animals. Bones protect the various other organs of the body, produce red and white blood cells, store minerals, provide structure and support for the body, an ...
is hydroxyapatite
Hydroxyapatite, also called hydroxylapatite (HA), is a naturally occurring mineral form of calcium apatite with the formula Ca5(PO4)3(OH), but it is usually written Ca10(PO4)6(OH)2 to denote that the crystal unit cell comprises two entities ...
(ca. 70%) (mineralized calcium phosphate
The term calcium phosphate refers to a family of materials and minerals containing calcium ions (Ca2+) together with inorganic phosphate anions. Some so-called calcium phosphates contain oxide and hydroxide as well. Calcium phosphates are wh ...
). Apart from bone targeting, this polymer has been modified for other potential biomedical applications such as drug delivery
Drug delivery refers to approaches, formulations, manufacturing techniques, storage systems, and technologies involved in transporting a pharmaceutical compound to its target site to achieve a desired therapeutic effect. Principles related to dr ...
, surface coating, DNA delivery, mucoadhesion, and beyond.
See also
*Polyaspartic Polyaspartic ester chemistry was first introduced in the early 1990s making it a relatively new technology. The patents were issued to Bayer in Germany and Miles Corporation in the United States. It utilizes the aza-Michael addition reaction. These ...
References
{{Reflist, refs= {{cite book , first1= Kirt W. , last1= Rusenko , first2= Julie E. , last2= Donachy , first3= A. P. , last3= Wheeler , editor1-first= C. Steven , editor1-last= Sikes , editor2-first= A. P. , editor2-last= Wheeler , year= 1991 , title= Surface Reactive Peptides and Polymers , doi= 10.1021/bk-1991-0444.ch008 , series = ACS Symposium Series , volume = 444 , chapter = Purification And Characterization Of A Shell Matrix Phosphoprotein From The American Oyster , publisher= ACS , pages= 107–124 , isbn= 9780841218864 {{cite book , first1= Winfried , last1= Joentgen , first2= Nikolaus , last2= Müller , first3= Alfred , last3= Mitschker , first4= Holger , last4= Schmidt , editor1-first= Stephen , editor1-last= Fahnestock , editor2-first= Alexander , editor2-last= Steinbüchel , authorlink= , title= Polyamides and Complex Proteinaceous Materials I , volume =7 , series = Biopolymers , chapter-url= http://www.wiley-vch.de/books/biopoly/con_v07.html , year= 2004 , publisher= Wiley-VCH , pages= 175–179 , chapter= Polyaspartic acids , isbn= 9783527302222 {{cite journal , first1= Hugo , last1= Schiff , year= 1897 , title= Ueber Polyaspartsäuren , journal= Ber. Dtsch. Chem. Ges. , volume= 30 , issue= 3 , language= german , pages= 2449–2459 , doi= 10.1002/cber.18970300316 {{cite journal , first1= J. , last1= Kovács , first2= I. , last2= Könyves , first3=Á. , last3= Pusztai , year= 1953 , title= Darstellung von Polyasparaginsäuren (Polyaspartsäuren) aus dem thermischen Autokondensationsprodukt der Asparaginsäure , journal= Experientia , volume= 9 , issue= 12 , language= german , pages=459–460 , doi= 10.1007/BF02165821 , pmid= 13127859 , s2cid= 40153017 {{cite journal , first1= G. D. , last1= Bennett , title= A Green Polymerization of Aspartic Acid for the Undergraduate Organic Laboratory , journal= Journal of Chemical Education , year= 2005 , volume= 82 , issue= 9 , pages= 1380–1381 , doi= 10.1021/ed082p1380 , bibcode = 2005JChEd..82.1380B {{cite journal , first1= J. , last1= Kovács , first2= I. , last2= Könyves , year= 1954 , title= Uber DL-α,β-Polyasparaginsaure , journal= Naturwissenschaften , volume= 41 , issue= 14 , language= german , pages=333 , doi= 10.1007/BF00644501 , bibcode = 1954NW.....41..333K , s2cid= 33648417 {{cite book , first1= Kim C. , last1= Low , first2= A. P. , last2= Wheeler , first3= Larry P. , last3= Koskan , editor1-first= J. Edward , editor1-last= Glass , year= 1996 , title= Hydrophilic Polymers , doi= 10.1021/ba-1996-0248.ch006 , series= Advances in Chemistry , volume= 248 , chapter= 6: Commercial Poly(aspartic acid) and Its Uses , publisher= ACS , pages99–111
, isbn= 9780841231337 , chapter-url-access= registration , chapter-url= https://archive.org/details/hydrophilicpolym0000unse , url= https://archive.org/details/hydrophilicpolym0000unse/page/99 {{cite journal , first1= H. , last1= Pivcova , first2= V. , last2= Saudek , first3= J. , last3= Drobnik , first4= J. , last4= Vlasak , year= 1981 , title= NMR Study of Poly(aspartic acid). I. α-and β-Peptide Bonds in Poly(aspartic acid) Prepared by Thermal Polycondensation , journal= Biopolymers , volume= 20 , issue= 8 , pages=1605–1614 , doi= 10.1002/bip.1981.360200804 , s2cid= 85201969 {{cite journal , first1= Etso , last1= Kokufuta , first2= Shinnichiro , last2= Suzuki , first3= Kaoru , last3= Harad , year= 1978 , title= Temperature Effect on the Molecular Weight and the Optical Purity of Anhydropolyaspartic Acid Prepared by Thermal Polycondensation , journal= Bulletin of the Chemical Society of Japan , volume= 51 , issue= 5 , pages=1555–1556 , doi= 10.1246/bcsj.51.1555 , doi-access= free {{cite journal , first1= Takeshi , last1= Nakato , first2= Atsushi , last2= Kusuno , first3= Toyoji , last3= Kakuchi , year= 2000 , title= Synthesis of poly(succinimide) by bulk polycondensation of L-aspartic acid with an acid catalyst , journal= Journal of Polymer Science Part A: Polymer Chemistry , volume= 38 , issue= 1 , pages=117–122 , doi= 10.1002/(SICI)1099-0518(20000101)38:1<117::AID-POLA15>3.0.CO;2-F , bibcode= 2000JPoSA..38..117N , doi-access= free {{cite journal , first1= Yaquan , last1= Wang , first2= Yongjiang , last2= Hou , first3= Gang , last3= Ruan , first4= Ming , last4= Pan , first5= Tengfei , last5= Liu , year= 2003 , title= Study on the polymerization of aspartic acid catalyzed by phosphoric acid , journal= Journal of Macromolecular Science-Pure and Applied Chemistry , volume= A40 , issue= 3 , pages=293–307 , doi= 10.1081/MA-120018116 , s2cid= 85163135 {{cite journal , first1= Vanga S. , last1= Rao , first2= Philippe , last2= Lapointe , first3= Donald N. , last3= McGregor , year= 1993 , title= Temperature Effect on the Molecular Weight and the Optical Purity of Anhydropolyaspartic Acid Prepared by Thermal Polycondensation , journal= Makromolekulare Chemie-Macromolecular Chemistry and Physics , volume= 194 , issue= 4 , pages=1095–1104 , doi= 10.1002/macp.1993.021940405 {{cite journal , first1= Yasuyuki , last1= Soeda , first2= Kazunobu , last2= Toshima , first3= Shuichi , last3= Matsuma , year= 2003 , title= Sustainable enzymatic preparation of polyaspartate using a bacterial protease , journal= Biomacromolecules , volume= 4 , issue= 2 , pages=193–203 , doi= 10.1021/bm0200534 , pmid= 12625712 {{cite journal , first1= V. , last1= Saudek , first2= H. , last2= Pivcova , first3= J. , last3= Drobnik , year= 1981 , title= NMR Study of Poly(aspartic acid). II. α-and β-Peptide Bonds in Poly(aspartic acid) Prepared by Common Methods , journal= Biopolymers , volume= 20 , issue= 8 , pages=1615–1623 , doi= 10.1002/bip.1981.360200805 , s2cid= 84203387 {{cite patent , country = US , number = 5468838 , status = patent , title = Polysuccinimide, polyaspartic acid and their salts are prepared by reaction of maleic anhydride and ammonia, polycondensation of the resulting product in the presence of a solubilizing agent and, if appropriate, hydrolysis. , pubdate = 1995-11-21 , gdate = , fdate = 1994-03-14 , pridate = , invent1 = Boehmke, Gunter , invent2 = Schmitz, Gerd , assign1 = Bayer AG , assign2 = , class = {{Cite journal , last1 = Gross , first1 = Richard A. , last2 = Kalra , first2 = Bhanu , year = 2002 , title = Biodegradable Polymers for the Environment , journal = Science , volume = 297 , issue = 5582 , pages = 803–807 , doi = 10.1126/science.297.5582.803 , pmid = 12161646 , bibcode=2002Sci...297..803G, url = https://zenodo.org/record/1231185 {{Cite journal , first1= David , last1= Hasson , first2= Hilla , last2= Shemer , first3= Alexander , last3= Sher , year= 2011 , title= State of the Art of Friendly "Green" Scale Control Inhibitors: A Review Article , journal= Industrial & Engineering Chemistry Research , volume= 50 , issue= 12 , pages=7601–7607 , doi= 10.1021/ie200370v {{Cite journal , first1= M. J. , last1= Zahuriaan-Mehr , first2= A. , last2= Pourjavadi , first3= H. , last3= Salimi , first4= M. , last4= Kurdtabar , year= 2009 , title= Protein- and homo poly(amino acid)-based hydrogels with super-swelling properties , journal= Polymers for Advanced Technologies , volume= 20 , issue= 8 , pages=655–671 , doi= 10.1002/pat.1395 {{Cite journal , last1 = Thombre , first1 = Sunita M. , last2 = Sarwade , first2 = Bhimaro D. , year = 2005 , title = Synthesis and Biodegradability of Polyaspartic Acid: A Critical Review , journal = Journal of Macromolecular Science, Part A , volume = 42 , issue = 9 , pages = 1299–1315 , url = http://www.informaworld.com/index/718581646.pdf , doi = 10.1080/10601320500189604 , s2cid = 94818855 , 2 Polyamides Polyelectrolytes Chelating agents