Peterson Reaction on:
[Wikipedia]
[Google]
[Amazon]
The Peterson olefination (also called the Peterson reaction) is the 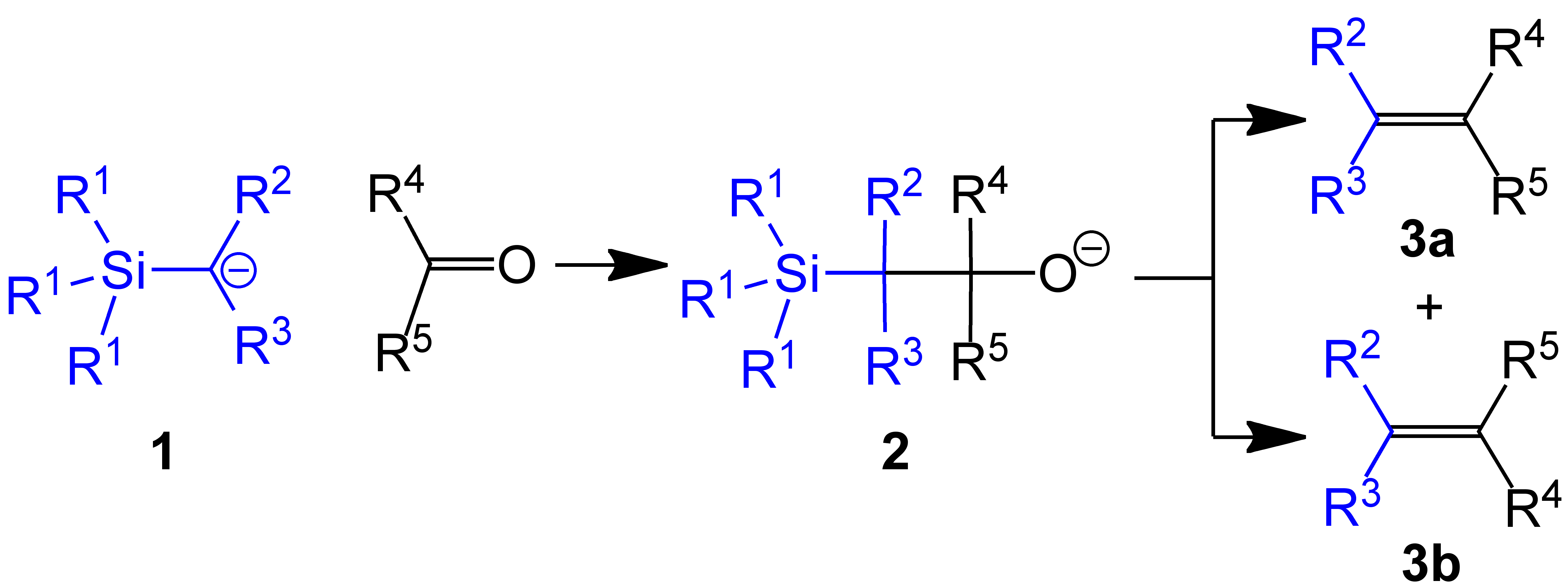 Several reviews have been published.Ager, D. J. ''Org. React.'' 1990, ''38'', 1.
Several reviews have been published.Ager, D. J. ''Org. React.'' 1990, ''38'', 1.


chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
of α-silyl carbanions (1 in diagram below) with ketones (or aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
s) to form a β-hydroxysilane (2) which eliminates to form alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
s (3).
 Several reviews have been published.Ager, D. J. ''Org. React.'' 1990, ''38'', 1.
Several reviews have been published.Ager, D. J. ''Org. React.'' 1990, ''38'', 1.
Reaction mechanism
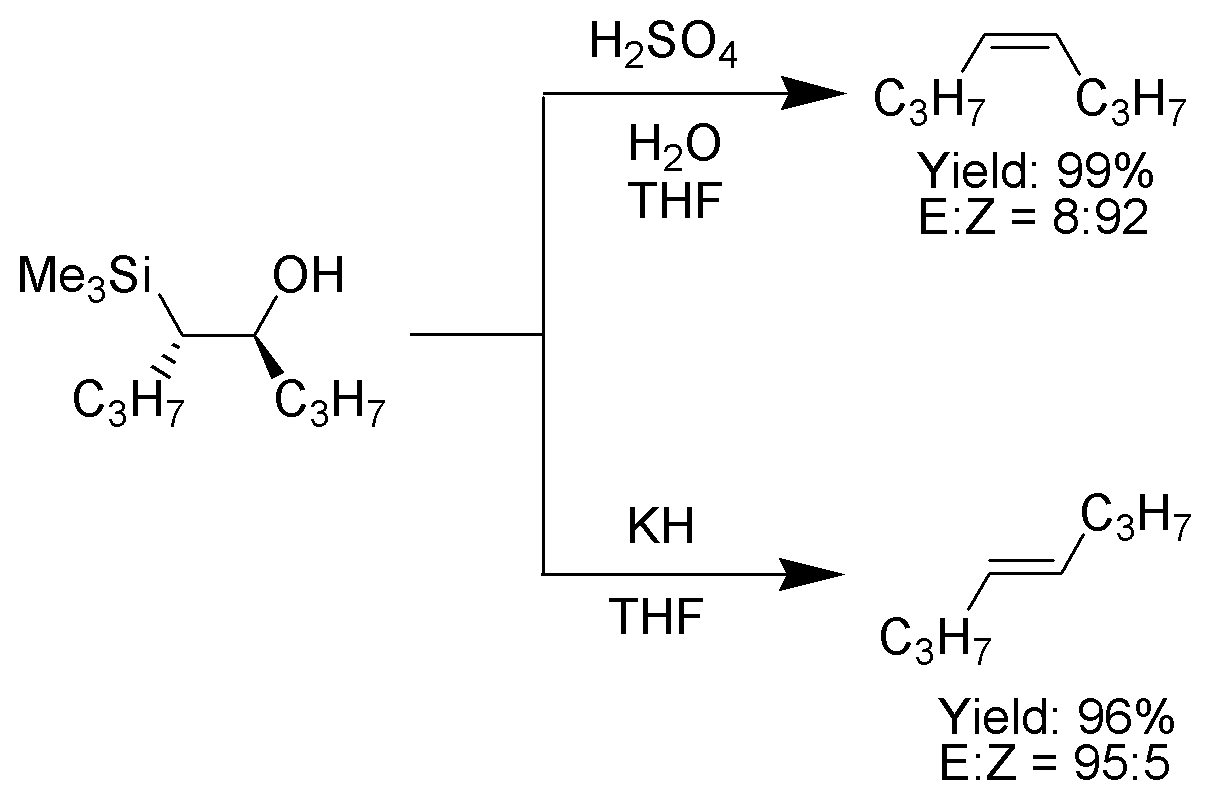
One attractive feature of the Peterson olefination is that it can be used to prepare either cis- or trans-alkenes from the same β-hydroxysilane. Treatment of the β-hydroxysilane with acid will yield one alkene, while treatment of the same β-hydroxysilane with base will yield the alkene of opposite stereochemistry.Basic elimination
The action of base upon a β-hydroxysilane (1) results in a concerted ''syn'' elimination of (2) or (3) to form the desired alkene. The penta-coordinate silicate intermediate (3) is postulated, but no proof exists to date.
Potassium
Potassium is the chemical element with the symbol K (from Neo-Latin ''kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmosph ...
alkoxides eliminate quickly, while sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable ...
alkoxides generally require heating. Magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ta ...
alkoxides only eliminate in extreme conditions. The order of reactivity of alkoxides, K > Na >> Mg, is consistent with higher electron density on oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
, hence increasing the alkoxide nucleophilicity.
Acidic elimination
The treatment of the β-hydroxysilane (1) with acid results in protonation and an ''anti'' elimination to form the desired alkene.
Alkyl substituents
When the α-silyl carbanion contains onlyalkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloa ...
, hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
, or electron-donating substituents, the stereochemical
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereois ...
outcome of the Peterson olefination can be controlled, because at low temperature the elimination is slow and the intermediate β-hydroxysilane can be isolated.
Once isolated, the diastereomeric β-hydroxysilanes are separated. One diastereomer
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have di ...
is treated with acid, while the other is treated with base, thus converted the material to an alkene with the required stereochemistry.

Electron-withdrawing substituents
When the α-silyl carbanion contains electron-withdrawing substituents, the Peterson olefination directly forms the alkene. The intermediate β-hydroxysilane cannot be isolated as it eliminates ''in-situ''. The basic elimination pathway has been postulated in these cases.Variations
Acidic elimination conditions are sometimes not feasible as the acid also promotes double bondisomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomeriz ...
. Additionally, elimination using sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable ...
or potassium hydride
Potassium hydride, KH, is the inorganic compound of potassium and hydrogen. It is an alkali metal hydride. It is a white solid, although commercial samples appear gray. It is a powerful superbase that is useful in organic synthesis. It is sold c ...
may not be feasible due to incompatible functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the re ...
s. Chan ''et al.'' have found that acylation of the intermediate silylcarbinol with either acetyl chloride
Acetyl chloride (CH3COCl) is an acyl chloride derived from acetic acid. It belongs to the class of organic compounds called acid halides. It is a colorless, corrosive, volatile liquid. Its formula is commonly abbreviated to AcCl.
Synthesis
On a ...
or thionyl chloride
Thionyl chloride is an inorganic compound with the chemical formula . It is a moderately volatile, colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a chlorinating reagent, with approximately per year bein ...
gives a β-silyl ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides a ...
that will eliminate spontaneously at 25 °C giving the desired alkene. Corey and co-workers developed a method (sometimes dubbed the ''Corey-Peterson olefination'') using a silylated imine to yield an α,β-unsaturated aldehyde from a carbonyl compound in one step. For an example for its use in total synthesis see: Kuwajima Taxol total synthesis The Kuwajima Taxol total synthesis by the group of Isao Kuwajima of the Tokyo Institute of Technology is one of several efforts in taxol total synthesis published in the 1990s. The total synthesis of Taxol is considered a landmark in organic synt ...
See also
*Horner–Wadsworth–Emmons reaction
The Horner–Wadsworth–Emmons (HWE) reaction is a chemical reaction used in organic chemistry of stabilized phosphonate carbanions with aldehydes (or ketones) to produce predominantly E-alkenes.
In 1958, Leopold Horner published a modifi ...
* Tebbe olefination
* Wittig reaction
The Wittig reaction or Wittig olefination is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide called a Wittig reagent. Wittig reactions are most commonly used to convert aldehydes and ketones to alkenes. Mos ...
References
{{Alkenes Olefination reactions Carbon-carbon bond forming reactions Substitution reactions Name reactions