Petersen Olefination on:
[Wikipedia]
[Google]
[Amazon]
The Peterson olefination (also called the Peterson reaction) is the chemical reaction of α-silyl carbanions (1 in diagram below) with 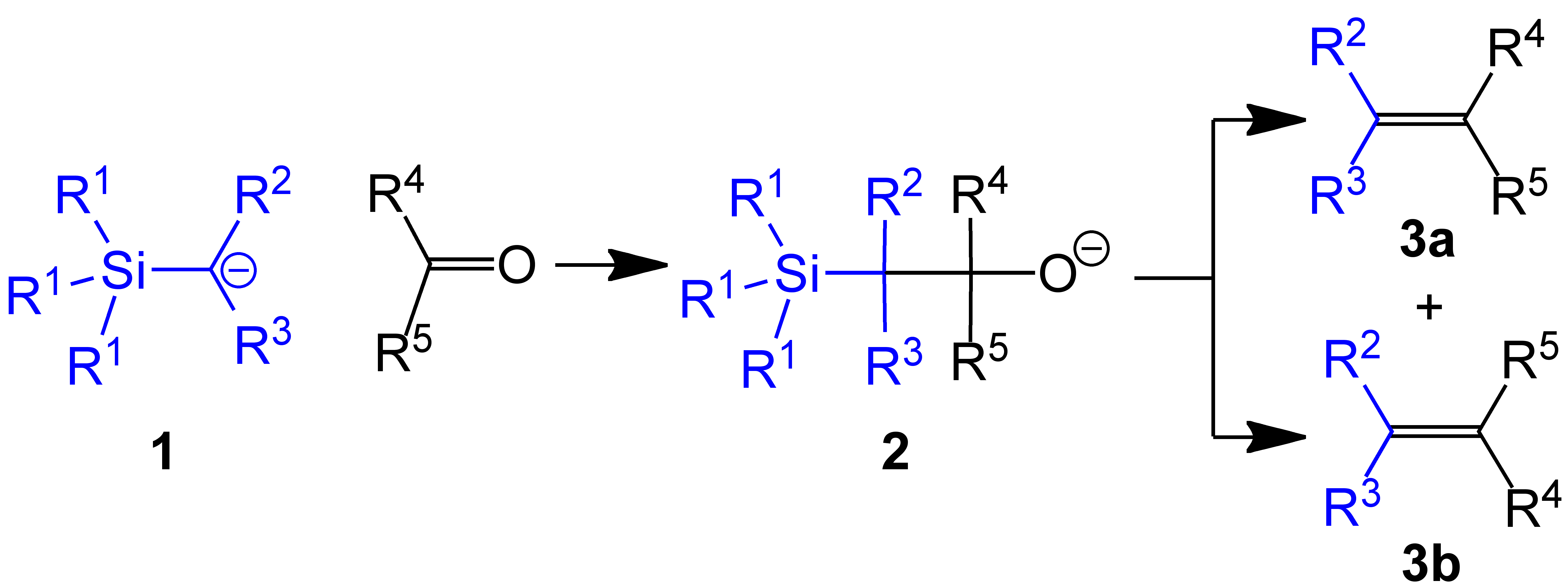 Several reviews have been published.Ager, D. J. ''Org. React.'' 1990, ''38'', 1.
Several reviews have been published.Ager, D. J. ''Org. React.'' 1990, ''38'', 1.
 Potassium alkoxides eliminate quickly, while sodium alkoxides generally require heating. Magnesium alkoxides only eliminate in extreme conditions. The order of reactivity of alkoxides, K > Na >> Mg, is consistent with higher electron density on oxygen, hence increasing the alkoxide nucleophilicity.
Potassium alkoxides eliminate quickly, while sodium alkoxides generally require heating. Magnesium alkoxides only eliminate in extreme conditions. The order of reactivity of alkoxides, K > Na >> Mg, is consistent with higher electron density on oxygen, hence increasing the alkoxide nucleophilicity.

ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
s (or aldehydes) to form a β-hydroxysilane (2) which eliminates to form alkenes (3).
 Several reviews have been published.Ager, D. J. ''Org. React.'' 1990, ''38'', 1.
Several reviews have been published.Ager, D. J. ''Org. React.'' 1990, ''38'', 1.
Reaction mechanism
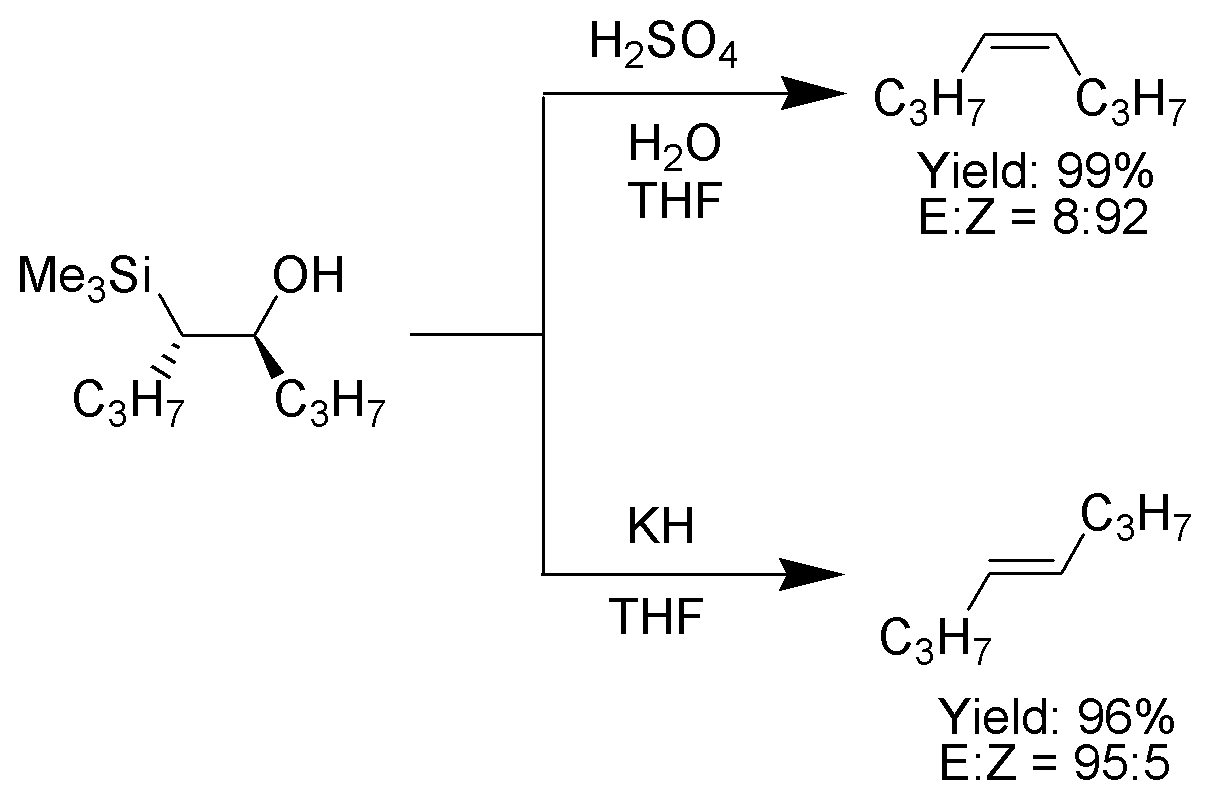
One attractive feature of the Peterson olefination is that it can be used to prepare either cis- or trans-alkenes from the same β-hydroxysilane. Treatment of the β-hydroxysilane with acid will yield one alkene, while treatment of the same β-hydroxysilane with base will yield the alkene of opposite stereochemistry.Basic elimination
The action of base upon a β-hydroxysilane (1) results in a concerted ''syn'' elimination of (2) or (3) to form the desired alkene. The penta-coordinatesilicate
In chemistry, a silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate (), metasilicate (), and pyrosilicate (, ). The name is al ...
intermediate (3) is postulated, but no proof exists to date.
 Potassium alkoxides eliminate quickly, while sodium alkoxides generally require heating. Magnesium alkoxides only eliminate in extreme conditions. The order of reactivity of alkoxides, K > Na >> Mg, is consistent with higher electron density on oxygen, hence increasing the alkoxide nucleophilicity.
Potassium alkoxides eliminate quickly, while sodium alkoxides generally require heating. Magnesium alkoxides only eliminate in extreme conditions. The order of reactivity of alkoxides, K > Na >> Mg, is consistent with higher electron density on oxygen, hence increasing the alkoxide nucleophilicity.
Acidic elimination
The treatment of the β-hydroxysilane (1) with acid results in protonation and an ''anti'' elimination to form the desired alkene.
Alkyl substituents
When the α-silyl carbanion contains only alkyl, hydrogen, or electron-donating substituents, thestereochemical
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereois ...
outcome of the Peterson olefination can be controlled, because at low temperature the elimination is slow and the intermediate β-hydroxysilane can be isolated.
Once isolated, the diastereomeric β-hydroxysilanes are separated. One diastereomer is treated with acid, while the other is treated with base, thus converted the material to an alkene with the required stereochemistry.

Electron-withdrawing substituents
When the α-silyl carbanion contains electron-withdrawing substituents, the Peterson olefination directly forms the alkene. The intermediate β-hydroxysilane cannot be isolated as it eliminates ''in-situ''. The basic elimination pathway has been postulated in these cases.Variations
Acidic elimination conditions are sometimes not feasible as the acid also promotes double bond isomerization. Additionally, elimination using sodium orpotassium hydride
Potassium hydride, KH, is the inorganic compound of potassium and hydrogen. It is an alkali metal hydride. It is a white solid, although commercial samples appear gray. It is a powerful superbase that is useful in organic synthesis. It is sold com ...
may not be feasible due to incompatible functional groups. Chan ''et al.'' have found that acylation of the intermediate silylcarbinol with either acetyl chloride or thionyl chloride gives a β-silyl ester that will eliminate spontaneously at 25 °C giving the desired alkene. Corey and co-workers developed a method (sometimes dubbed the ''Corey-Peterson olefination'') using a silylated imine to yield an α,β-unsaturated aldehyde from a carbonyl compound in one step. For an example for its use in total synthesis see: Kuwajima Taxol total synthesis
See also
*Horner–Wadsworth–Emmons reaction
The Horner–Wadsworth–Emmons (HWE) reaction is a chemical reaction used in organic chemistry of stabilized phosphonate carbanions with aldehydes (or ketones) to produce predominantly E-alkenes.
In 1958, Leopold Horner published a modifi ...
* Tebbe olefination
Tebbe's reagent is the organometallic compound with the formula (C5H5)2TiCH2ClAl(CH3)2. It is used in the methylenation of carbonyl compounds, that is it converts organic compounds containing the R2C=O group into the related R2C=CH2 derivative. It ...
* Wittig reaction
References
{{Alkenes Olefination reactions Carbon-carbon bond forming reactions Substitution reactions Name reactions