Patchoulol on:
[Wikipedia]
[Google]
[Amazon]
Patchoulol or patchouli alcohol (C15H26O) is a
 A serendipitous finding by Dunitz and co-workers revealed a contradictory structure. They had undertaken an X-ray analysis of the patchouli alcohol diester of
A serendipitous finding by Dunitz and co-workers revealed a contradictory structure. They had undertaken an X-ray analysis of the patchouli alcohol diester of 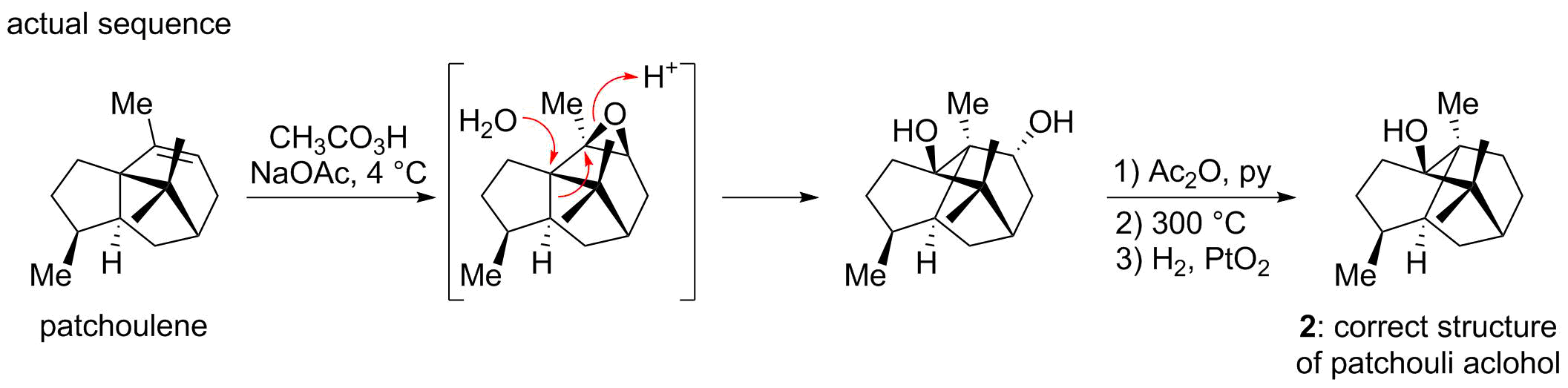 Contains embedded bicyclo .2.2ctane motif.
Contains embedded bicyclo .2.2ctane motif.
Chime 3D representation
Perfume ingredients Secondary alcohols Sesquiterpenes
sesquiterpene
Sesquiterpenes are a class of terpenes that consist of three isoprene units and often have the molecular formula C15H24. Like monoterpenes, sesquiterpenes may be cyclic or contain rings, including many unique combinations. Biochemical modificatio ...
alcohol found in patchouli
PatchouliAlso spelled ''patchouly'' or ''pachouli''. (; ''Pogostemon cablin'') is a species of flowering plant in the family Lamiaceae, commonly called the mint or deadnettle family. The plant grows as a bushy perennial herb, with erect stems r ...
. Patchouli oil is an important material in perfumery. The (−)-optical isomer
In chemistry, a molecule or ion is called chiral () if it cannot be superposed on its mirror image by any combination of rotations, translations, and some conformational changes. This geometric property is called chirality (). The terms are d ...
is one of the organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The ...
s responsible for the typical patchouli scent. Patchoulol is also used in the synthesis of the chemotherapy drug Taxol
Paclitaxel (PTX), sold under the brand name Taxol among others, is a chemotherapy medication used to treat a number of types of cancer. This includes ovarian cancer, esophageal cancer, breast cancer, lung cancer, Kaposi's sarcoma, cervical cance ...
.
Structure determination
Patchouli alcohol was first isolated in 1869 by Gal and its chemical composition later correctly formulated as C15H26O by Montgolfier. During early structural investigation the presence of a saturated tricyclic tertiary alcohol was established. After several years of careful study Büchi and co-workers proposed the structure of patchouli alcohol to correspond to 1, based on degradation studies from his earlier work, verified later by synthesis of material which corresponded to the natural authentic sample of patchouli alcohol. A serendipitous finding by Dunitz and co-workers revealed a contradictory structure. They had undertaken an X-ray analysis of the patchouli alcohol diester of
A serendipitous finding by Dunitz and co-workers revealed a contradictory structure. They had undertaken an X-ray analysis of the patchouli alcohol diester of chromic acid
The term chromic acid is usually used for a mixture made by adding concentrated sulfuric acid to a dichromate, which may contain a variety of compounds, including solid chromium trioxide. This kind of chromic acid may be used as a cleaning mixt ...
, with the objective of determining the Cr-O-C angles. In the course of their analysis they found that the X-ray evidence could not be reconciled with the proposed structure 1. Instead they proposed together with Büchi the novel structure 2. The discrepancy had resulted from an unanticipated skeletal rearrangement that had occurred in the Büchi synthesis when patchoulene was treated with peroxy acid
A peroxy acid (often spelled as one word, peroxyacid, and sometimes called peracid) is an acid which contains an acidic –OOH group. The two main classes are those derived from conventional mineral acids, especially sulfuric acid, and the peroxy ...
, an operation that by accident generated the correct architecture of the natural product.
 Contains embedded bicyclo .2.2ctane motif.
Contains embedded bicyclo .2.2ctane motif.
See also
*Norpatchoulenol
Norpatchoulenol is a tricyclic terpenoid found in commercial patchouli extract in small quantities, and thought to contribute significantly to the aroma of patchouli oil.
See also
*Patchoulol
Patchoulol or patchouli alcohol (C15H26O) is a sesqu ...
References
{{ReflistExternal links
Chime 3D representation
Perfume ingredients Secondary alcohols Sesquiterpenes