optical isomerism on:
[Wikipedia]
[Google]
[Amazon]
 In
In
 The asymmetric atom is called a chirality center, a type of
The asymmetric atom is called a chirality center, a type of
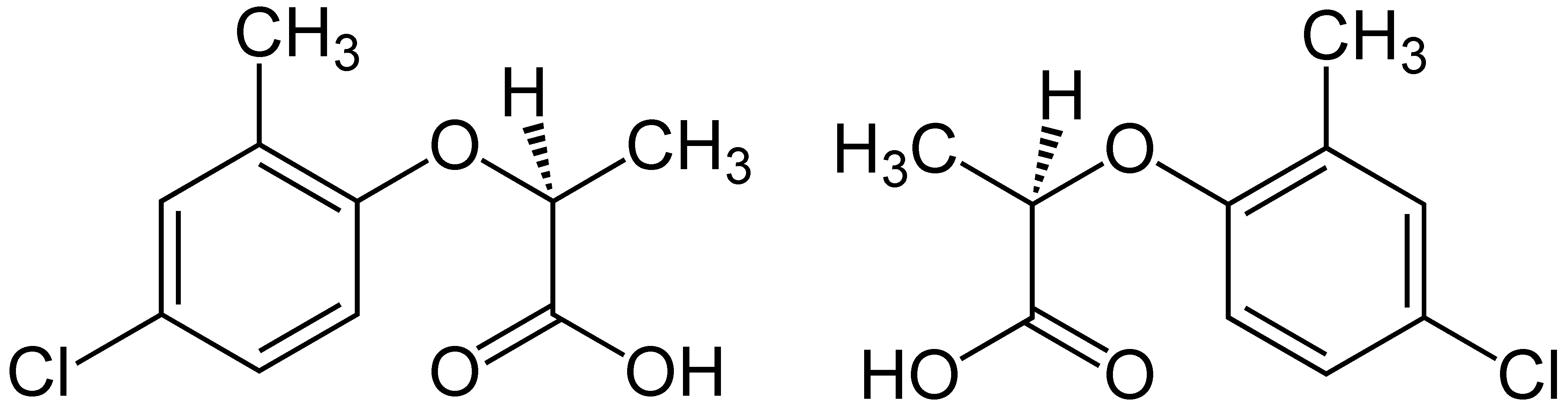 An example of such an enantiomer is the
An example of such an enantiomer is the
chemwiki:stereoisomerism
{{Chiral synthesis Stereochemistry Isomerism
chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds made of atoms, molecules and ions: their composition, structure, proper ...
, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek
Ancient Greek includes the forms of the Greek language used in ancient Greece and the ancient world from around 1500 BC to 300 BC. It is often roughly divided into the following periods: Mycenaean Greek (), Dark Ages (), the Archaic pe ...
ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical antipode – is one of two stereoisomer
In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in ...
s that are non-superposable onto their own mirror image
A mirror image (in a plane mirror) is a reflected duplication of an object that appears almost identical, but is reversed in the direction perpendicular to the mirror surface. As an optical effect it results from reflection off from substance ...
. Enantiomers are much like one's right and left hands, when looking at the same face, they cannot be superposed onto each other. No amount of reorientation will allow the four unique groups on the chiral carbon (see Chirality (chemistry)
In chemistry, a molecule or ion is called chiral () if it cannot be superposed on its mirror image by any combination of rotations, translations, and some conformational changes. This geometric property is called chirality (). The terms a ...
) to line up exactly. The number of stereoisomers a molecule has can be determined by the number of chiral carbons it has. Stereoisomers include both enantiomers and diastereomers.
Diastereomer
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have di ...
s, like enantiomers, share the same molecular formula and are non-superposable onto each other however, they are not mirror images of each other.
A molecule with chirality rotates plane-polarized light. A mixture of equals amounts of each enantiomer, a ''racemic mixture
In chemistry, a racemic mixture, or racemate (), is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as racemates. ...
'' or a ''racemate'', does not rotate light.
Naming conventions
There are three common naming conventions for specifying one of the two enantiomers (theabsolute configuration
Absolute configuration refers to the spatial arrangement of atoms within a chiral molecular entity (or group) and its resultant stereochemical description. Absolute configuration is typically relevant in organic molecules, where carbon is bonde ...
) of a given chiral molecule: the R/S system is based on the geometry of the molecule; the (+)- and (−)- system (also written using the obsolete equivalents ''d''- and ''l''-) is based on its optical rotation
Optical rotation, also known as polarization rotation or circular birefringence, is the rotation of the orientation of the plane of polarization about the optical axis of linearly polarized light as it travels through certain materials. Circul ...
properties; and the D/L system is based on the molecule's relationship to enantiomers of glyceraldehyde
Glyceraldehyde (glyceral) is a triose monosaccharide with chemical formula C3 H6 O3. It is the simplest of all common aldoses. It is a sweet, colorless, crystalline solid that is an intermediate compound in carbohydrate metabolism. The word comes ...
.
The R/S system is based on the molecule's geometry with respect to a chiral center. The R/S system is assigned to a molecule based on the priority rules assigned by Cahn–Ingold–Prelog priority rules
In organic chemistry, the Cahn–Ingold–Prelog (CIP) sequence rules (also the CIP priority convention; named for R.S. Cahn, C.K. Ingold, and Vladimir Prelog) are a standard process to completely and unequivocally name a stereoisomer of a ...
, in which the group or atom with the largest atomic number is assigned the highest priority and the group or atom with the smallest atomic number is assigned the lowest atomic number.
The (+)- and (−)- is used to specify a molecule's optical rotation
Optical rotation, also known as polarization rotation or circular birefringence, is the rotation of the orientation of the plane of polarization about the optical axis of linearly polarized light as it travels through certain materials. Circul ...
— the direction that the molecule in rotates polarized light. When a molecule is denoted dextrorotatory it is rotating the plane of polarized light clockwise and can also be denoted as (+). When it is denoted as levorotatory it is rotating the plane of polarized light counterclockwise and can also be denoted as (−).
The Latin words for ''left'' are ''laevus'' and ''sinister'', and the word for ''right'' is ''dexter'' (or ''rectus'' in the sense of correct or virtuous). The English word ''right'' is a cognate
In historical linguistics, cognates or lexical cognates are sets of words in different languages that have been inherited in direct descent from an etymological ancestor in a common parent language. Because language change can have radical ef ...
of ''rectus''. This is the origin of the L/D and S/R notations, and the employment of prefixes ''levo-'' and ''dextro-'' in common names
In biology, a common name of a taxon or organism (also known as a vernacular name, English name, colloquial name, country name, popular name, or farmer's name) is a name that is based on the normal language of everyday life; and is often contrast ...
.
The prefix ''ar-'', from the Latin ''recto'' (right), is applied to the right-handed version; ''es-'', from the Latin ''sinister'' (left), to the left-handed molecule. Example: ketamine
Ketamine is a dissociative anesthetic used medically for induction and maintenance of anesthesia. It is also used as a recreational drug. It is one of the safest anesthetics, as, in contrast with opiates, ether, and propofol, it suppress ...
, arketamine
Arketamine (developmental code names PCN-101, HR-071603), also known as (''R'')-ketamine or (''R'')-(−)-ketamine, is the (''R'')-(−) enantiomer of ketamine. Similarly to racemic ketamine and esketamine, the ''S''(+) enantiomer of k ...
, esketamine.
Chirality centers
stereocenter
In stereochemistry, a stereocenter of a molecule is an atom (center), axis or plane that is the focus of stereoisomerism; that is, when having at least three different groups bound to the stereocenter, interchanging any two different groups c ...
. A chirality center is also called a ''chiral center'' or an ''asymmetric center''. Some sources use the terms ''stereocenter'', ''stereogenic center'', ''stereogenic atom'' or ''stereogen'' to refer exclusively to a chirality center, while others use the terms more broadly to refer also to centers that result in diastereomers
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have di ...
(stereoisomers that are not enantiomers).
Compounds that contain exactly one (or any odd number) of asymmetric atoms are always chiral. However, compounds that contain an even number of asymmetric atoms sometimes lack chirality because they are arranged in mirror-symmetric pairs, and are known as ''meso'' compounds. For instance, ''meso'' tartaric acid
Tartaric acid is a white, crystalline organic acid that occurs naturally in many fruits, most notably in grapes, but also in bananas, tamarinds, and citrus. Its salt, potassium bitartrate, commonly known as cream of tartar, develops naturally ...
(shown on the right) has two asymmetric carbon atoms, but it does not exhibit enantiomerism because there is a mirror symmetry plane. Conversely, there exist forms of chirality that do not require asymmetric atoms, such as axial
Axial may refer to:
* one of the anatomical directions describing relationships in an animal body
* In geometry:
:* a geometric term of location
:* an axis of rotation
* In chemistry, referring to an axial bond
* a type of modal frame, in music
* ...
, planar, and helical chirality.
Even though a chiral molecule lacks reflection (Cs) and rotoreflection
In geometry, an improper rotation,. also called rotation-reflection, rotoreflection, rotary reflection,. or rotoinversion is an isometry in Euclidean space that is a combination of a rotation about an axis and a reflection in a plane perpendicul ...
symmetries (S2''n''), it can have other molecular symmetries, and its symmetry is described by one of the chiral point groups
In geometry, a point group is a mathematical group of symmetry operations ( isometries in a Euclidean space) that have a fixed point in common. The coordinate origin of the Euclidean space is conventionally taken to be a fixed point, and every ...
: C''n'', D''n'', T, O, or I. For example, hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3 ...
is chiral and has C2 (two-fold rotational) symmetry. A common chiral case is the point group C1, meaning no symmetries, which is the case for lactic acid.
Examples
 An example of such an enantiomer is the
An example of such an enantiomer is the sedative
A sedative or tranquilliser is a substance that induces sedation by reducing irritability or excitement. They are CNS depressants and interact with brain activity causing its deceleration. Various kinds of sedatives can be distinguished, but ...
thalidomide
Thalidomide, sold under the brand names Contergan and Thalomid among others, is a medication used to treat a number of cancers (including multiple myeloma), graft-versus-host disease, and a number of skin conditions including complications o ...
, which was sold in a number of countries around the world from 1957 until 1961. It was withdrawn from the market when it was found to cause birth defects. One enantiomer caused the desirable sedative effects, while the other, unavoidably present in equal quantities, caused birth defects.
The herbicide
Herbicides (, ), also commonly known as weedkillers, are substances used to control undesired plants, also known as weeds.EPA. February 201Pesticides Industry. Sales and Usage 2006 and 2007: Market Estimates. Summary in press releasMain page f ...
mecoprop is a racemic mixture, with the (''R'')-(+)-enantiomer ("Mecoprop-P", "Duplosan KV") possessing the herbicidal activity.
Another example is the antidepressant drugs escitalopram
Escitalopram, sold under the brand names Lexapro and Cipralex, among others, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class. Escitalopram is mainly used to treat major depressive disorder and generalized anxiet ...
and citalopram
Citalopram, sold under the brand name Celexa among others, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class. It is used to treat major depressive disorder, obsessive compulsive disorder, panic disorder, and so ...
. Citalopram is a racemate
In chemistry, a racemic mixture, or racemate (), is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as racemates. ...
:1 mixture of (''S'')-citalopram and (''R'')-citalopram escitalopram ''S'')-citalopramis a pure enantiomer. The dosages for escitalopram are typically 1/2 of those for citalopram. Here, (S)-citalopram is called a chiral switch of Citalopram.
Chiral drugs
Enantiomers display distinct biological effects.Enantiopure medications
Advances in industrial chemical processes have made it economic for pharmaceutical manufacturers to take drugs that were originally marketed as aracemic mixture
In chemistry, a racemic mixture, or racemate (), is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as racemates. ...
and market the individual enantiomers. This strategy of marketing of a chiral specific drug from an already approved and existing racemic drug is normally done for better therapeutic efficacy. This kind of switching from a racemic drug to an enantiopure drug is called a chiral switch and the process is called chiral switching. In some cases, the enantiomers have genuinely different effects. An interesting case is that of Propoxyphene. The enantiomeric pair of propoxyphene is separately sold by Eli Lilly and company. One of the partner is dextropropoxyphene, an analgesic
An analgesic drug, also called simply an analgesic (American English), analgaesic (British English), pain reliever, or painkiller, is any member of the group of drugs used to achieve relief from pain (that is, analgesia or pain management). It ...
agent (Darvon) and the other is called levopropoxyphene
Levopropoxyphene is an antitussive. It is an optical isomer of dextropropoxyphene. The racemic mixture is called propoxyphene. Only the dextro-isomer (dextropropoxyphene) has an analgesic effect; the levo-isomer appears to exert only an antitussi ...
, an effective antitussive
Cold medicines are a group of medications taken individually or in combination as a treatment for the symptoms of the common cold and similar conditions of the upper respiratory tract. The term encompasses a broad array of drugs, including ...
(Novrad). It is interesting to note that the trade names of the drugs, DARVON and NOVRAD, also reflect the chemical mirror-image relationship. In other cases, there may be no clinical benefit to the patient. In some jurisdictions, single-enantiomer drugs are separately patentable from the racemic mixture. It is possible that only one of the enantiomers is active. Or, it may be that both are active, in which case separating the mixture has no objective benefits, but extends the drug's patentability.
Enantioselective preparations
In the absence of an effective enantiomeric environment (precursor
Precursor or Precursors may refer to:
* Precursor (religion), a forerunner, predecessor
** The Precursor, John the Baptist
Science and technology
* Precursor (bird), a hypothesized genus of fossil birds that was composed of fossilized parts of u ...
, chiral catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
, or kinetic resolution), separation of a racemic mixture into its enantiomeric components is impossible, although certain racemic mixtures spontaneously crystallize in the form of a ''racemic conglomerate'', in which crystals of the enantiomers are physically segregated and may be separated mechanically. However, most racemates will crystallize in crystals containing both enantiomers in a 1:1 ratio, arranged in a regular lattice.
In his pioneering work, Louis Pasteur
Louis Pasteur (, ; 27 December 1822 – 28 September 1895) was a French chemist and microbiologist renowned for his discoveries of the principles of vaccination, microbial fermentation and pasteurization, the latter of which was named afte ...
was able to isolate the isomers of tartaric acid
Tartaric acid is a white, crystalline organic acid that occurs naturally in many fruits, most notably in grapes, but also in bananas, tamarinds, and citrus. Its salt, potassium bitartrate, commonly known as cream of tartar, develops naturally ...
because they crystallize from solution as crystals each with a different symmetry and separate them with tweezers. This is known as chiral resolution and its one of the two main strategies for the preparation of enantiopure compounds. A less common method is by enantiomer self-disproportionation.
The second strategy is asymmetric synthesis: the use of various techniques to prepare the desired compound in high enantiomeric excess. Techniques encompassed include the use of chiral starting materials (chiral pool synthesis The chiral pool is a "collection of abundant enantiopure building blocks provided by nature" used in synthesis. In other words, a chiral pool would be a large quantity of common organic enantiomers. Contributors to the chiral pool are amino acids, s ...
), the use of chiral auxiliaries
In stereochemistry, a chiral auxiliary is a Stereogenic center, stereogenic group or unit that is temporarily incorporated into an organic compound in order to control the stereochemical outcome of the synthesis. The chirality present in the auxil ...
and chiral catalysts, and the application of asymmetric induction
In stereochemistry, asymmetric induction (also enantioinduction) describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the su ...
. The use of enzymes (biocatalysis
Biocatalysis refers to the use of living (biological) systems or their parts to speed up (catalyze) chemical reactions. In biocatalytic processes, natural catalysts, such as enzymes, perform chemical transformations on organic compounds. Both enz ...
) may also produce the desired compound.
A third strategy is Enantioconvergent synthesis, the synthesis of one enantiomer from a racemic precursor, utilizing both enantiomers. By making use of a chiral catalyist, both enantiomers of the reactant result in a single enantiomer of product.
Enantiomers may not be isolable if there is an accessible pathway for racemization (interconversion between enantiomorphs to yield a racemic mixture) at a given temperature and timescale. For example, amines with three distinct substituents are chiral, but with few exceptions (e.g. substituted ''N''-chloroaziridines), they rapidly undergo " umbrella inversion" at room temperature, leading to racemization. If the racemization is fast enough, the molecule can often be treated as an achiral, averaged structure.
Parity violation
For all intents and purposes, each enantiomer in a pair has the same energy. However, theoretical physics predicts that due toparity violation
In physics, a parity transformation (also called parity inversion) is the flip in the sign of ''one'' spatial coordinate. In three dimensions, it can also refer to the simultaneous flip in the sign of all three spatial coordinates (a point ref ...
of the weak nuclear force
In nuclear physics and particle physics, the weak interaction, which is also often called the weak force or weak nuclear force, is one of the four known fundamental interactions, with the others being electromagnetism, the strong interacti ...
(the only force in nature that can "tell left from right"), there is actually a ''minute'' difference in energy between enantiomers (on the order of 10−12 eV or 10−10 kJ/mol or less) due to the weak neutral current mechanism. This difference in energy is far smaller than energy changes caused by even small changes in molecular conformation, and far too small to measure by current technology, and is therefore chemically inconsequential. In the sense used by particle physicists, the "true" enantiomer of a molecule, which has exactly the same mass-energy content as the original molecule, is a mirror-image that is also ''built from antimatter'' (antiprotons, antineutrons, and positrons). Throughout this article, "enantiomer" is used only in the chemical sense of compounds of ordinary matter that are not superposable on their mirror image.
''Quasi''-enantiomers
''Quasi''-enantiomers are molecular species that are not strictly enantiomers, but behave as if they are. In ''quasi''-enantiomers majority of the molecule is reflected; however, an atom or group within the molecule is changed to a similar atom or group. ''Quasi''-enantiomers can also be defined as molecules that have the potential to become enantiomers if an atom or group in the molecule is replaced. An example of ''quasi''-enantiomers would (''S'')-bromobutane and (''R'')-iodobutane. Under normal conditions the enantiomers for (''S'')-bromobutane and (''R'')-iodobutane would (''R)-''bromobutane and (''S'')-iodobutane respectively. ''Quasi''-enantiomers would also produce quasi-racemates, which are similar to normal racemates (seeRacemic mixture
In chemistry, a racemic mixture, or racemate (), is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as racemates. ...
) in that they form an equal mixture of ''quasi''-enantiomers.
Though not considered actual enantiomers, the naming convention for ''quasi-enantiomers also'' follows the same trend as enantiomers when looking at (''R'') and (''S'') configurations - which are considered from a geometrical basis (see Cahn–Ingold–Prelog priority rules
In organic chemistry, the Cahn–Ingold–Prelog (CIP) sequence rules (also the CIP priority convention; named for R.S. Cahn, C.K. Ingold, and Vladimir Prelog) are a standard process to completely and unequivocally name a stereoisomer of a ...
).
''Quasi''-enantiomers have applications in parallel kinetic resolution.G.S. Coumbarides, M. Dingjan, J. Eames, A. Flinn, J. Northen and Y. Yohannes, Tetrahedron Lett. 46 (2005), p. 2897er
See also
* Chiral switch *Crystal system
In crystallography, a crystal system is a set of point groups (a group of geometric symmetries with at least one fixed point). A lattice system is a set of Bravais lattices. Space groups are classified into crystal systems according to their poin ...
* Enantiopure drug
* Atropisomer
Atropisomers are stereoisomers arising because of hindered rotation about a single bond, where energy differences due to steric strain or other contributors create a barrier to rotation that is high enough to allow for isolation of individual ...
* Chirotechnology
* Chirality (physics)
A chiral phenomenon is one that is not identical to its mirror image (see the article on mathematical chirality). The spin of a particle may be used to define a handedness, or helicity, for that particle, which, in the case of a massless particle ...
* Diastereomer
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have di ...
* Dynamic stereochemistry In chemistry, dynamic stereochemistry studies the effect of stereochemistry on the reaction rate of a chemical reaction. Stereochemistry is involved in:
* stereospecific reactions
* stereoselective or asymmetric reactions
* racemisation In chemist ...
* Epimer
In stereochemistry, an epimer is one of a pair of diastereomers. The two epimers have opposite configuration at only one stereogenic center out of at least two. All other stereogenic centers in the molecules are the same in each. Epimerization is ...
* Molecular symmetry
Molecular symmetry in chemistry describes the symmetry present in molecules and the classification of these molecules according to their symmetry. Molecular symmetry is a fundamental concept in chemistry, as it can be used to predict or explain m ...
* Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereoi ...
* Stereocenter
In stereochemistry, a stereocenter of a molecule is an atom (center), axis or plane that is the focus of stereoisomerism; that is, when having at least three different groups bound to the stereocenter, interchanging any two different groups c ...
References
External links
*chemwiki:stereoisomerism
{{Chiral synthesis Stereochemistry Isomerism