Orsat apparatus on:
[Wikipedia]
[Google]
[Amazon]
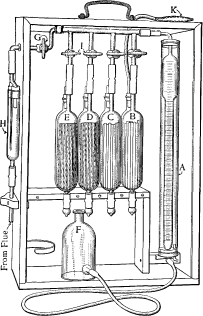 An Orsat gas analyser is a piece of
An Orsat gas analyser is a piece of
orsat apparatus
{{DEFAULTSORT:Orsat Gas Analyser Laboratory glassware
 An Orsat gas analyser is a piece of
An Orsat gas analyser is a piece of laboratory equipment
A laboratory (; ; colloquially lab) is a facility that provides controlled conditions in which scientific or technological research, experiments, and measurement may be performed. Laboratory services are provided in a variety of settings: physicia ...
used to analyse a gas sample (typically fossil fuel flue gas
Flue gas is the gas exiting to the atmosphere via a flue, which is a pipe or channel for conveying exhaust gases from a fireplace, oven, furnace, boiler or steam generator. Quite often, the flue gas refers to the combustion exhaust gas produc ...
) for its oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
, carbon monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simple ...
and carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is trans ...
content. Although largely replaced by instrumental techniques, the Orsat remains a reliable method of measurement and is relatively simple to use.
The apparatus was invented by Louis Orsat who reported it in the Annales des Mines in 1875. There was an earlier report by Thomas Egleston in 1873.
Construction
The apparatus consists of an intake valve which feeds into a calibrated water orglycerin
Glycerol (), also called glycerine in British English and glycerin in American English, is a simple triol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in lipids known ...
jacketed gas burette, this burette is then connected by tubing to two or more absorption pipettes containing chemical solutions that absorb the gases it is being used to measure. The intake and each of the absorption pipettes are valved with stopcocks to allow the movement of gas through the apparatus to be precisely controlled. For safety and portability, the apparatus is usually encased in a wooden box with a handle.
The most common absorbents are:
* Potassium Hydroxide
Potassium hydroxide is an inorganic compound with the formula K OH, and is commonly called caustic potash.
Along with sodium hydroxide (NaOH), KOH is a prototypical strong base. It has many industrial and niche applications, most of which exp ...
(Caustic Potash) for carbon dioxide
* Pyrogallol
Pyrogallol is an organic compound with the formula C6H3(OH)3. It is a water-soluble, white solid although samples are typically brownish because of its sensitivity toward oxygen. It is one of three isomers of benzenetriols.
Production and reac ...
(Pyrogallic Acid) for oxygen
* Copper(I) chloride
Copper(I) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula CuCl. The substance is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. Impure samples appear gre ...
(ammoniacal Cuprous chloride) for carbon monoxide
Any left over gas is assumed to be nitrogen, though other absorbents or vessels can be used to isolate additional gases. Platinum on asbestos for example can be used to determine the hydrogen content of a sample, and The Fischer type Orsat gas analyser for example uses a platinum electrode to explode the remaining gases with hydrogen.
The base of the gas burette is connected to a leveling bottle which typically contains slightly acidulated water with a trace of chemical indicator (typically methyl orange
Methyl orange is a pH indicator frequently used in titration because of its clear and distinct color variance at different pH values. Methyl orange shows red color in acidic medium and yellow color in basic medium. Because it changes color at the ...
) for colouration. The small amount of acid added to the water reduces the solubility of carbon dioxide. The leveling bottle can be lifted and lowered to enable readings to be taken at constant pressure and to transfer the gas to and from the pipettes containing the different absorption media. The movement of gas through the apparatus is entirely controlled using the leveling bottle and the various stopcocks.
Method of analysis
By means of arubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds. Thailand, Malaysia, an ...
tubing arrangement, the gas to be analyzed is drawn into the burette and flushed through several times. Using the stopcocks to isolate the absorption pipettes 100ml is typically withdrawn into the main burette for ease of calculation and the leveling flask is raised until the water is level between it and the burette. This insures that the sample is of a known volume and is in equilibrium with the pressure of the room. The water or glycerin jacket further assures that the sample is kept at room temperature.
The gas is then passed into the Potassium Hydroxide
Potassium hydroxide is an inorganic compound with the formula K OH, and is commonly called caustic potash.
Along with sodium hydroxide (NaOH), KOH is a prototypical strong base. It has many industrial and niche applications, most of which exp ...
(caustic potash) burette by opening the stop cock and lifting the leveling flask. This siphons water into the burette which pushes the gas into the absorption vessel. The gas is left to stand for about two minutes and then withdrawn, isolating the remaining gas via the stopcock arrangements. The process is then repeated to ensure full absorption. Afterward the leveling flask is once more adjusted until the fluid level is equal between both vessels and a measurement of the new gas volume is taken. If 100ml of gas was present initially the new volume indicates the percentage of carbon dioxide absorbed. If a sample after absorption contained 88ml of gas, then it would be recorded as 12% carbon dioxide.
The same technique is repeated for oxygen, using the pyrogallol, and carbon monoxide using the ammoniacal cuprous chloride though depending on any additional absorption media the process may be different. Potassium Hydroxide for example will also absorb sulfur dioxide, and so the step to measure SO2 would need to come first.
Other types
* Orsat-Aimer Products Orsat apparatus
* Orsat-Fischer apparatus
* Orsat-Lunge apparatus
* Orsat-Friedrichs apparatus
* Sona Orsat apparatus
* Fischer type Orsat gas analyser
References
* Boiler House and Power Station Chemistry: Wilfred Francis, 1955 * A Textbook of Quantitative Inorganic Analysis: Arthur I Vogel, 1961. * Burgess H. Jennings. ''Internal Combustion Engines Analysis and Practice'', 1944. * A. Meyer. ''The Combustion Gas Turbine. Mechanical Engineering'', Vol 61, 1939.External links
* Chemical Absorption Reaction iorsat apparatus
{{DEFAULTSORT:Orsat Gas Analyser Laboratory glassware