organocerium on:
[Wikipedia]
[Google]
[Amazon]
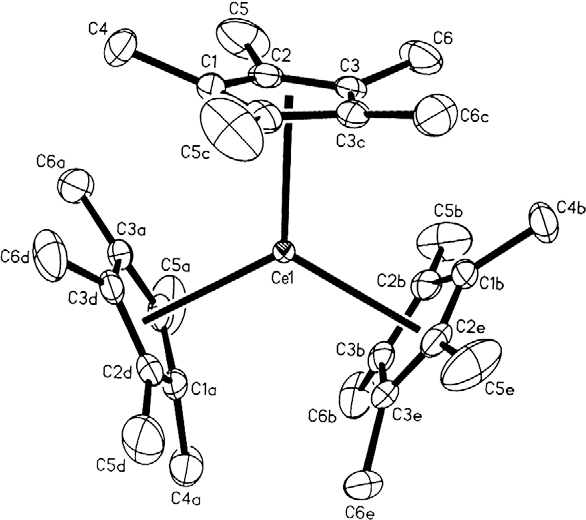 Organocerium chemistry is the science exploring the properties, structure, and reactivity of organocerium compounds,
Organocerium chemistry is the science exploring the properties, structure, and reactivity of organocerium compounds,

 Despite this high reactivity, organocerium reagents are almost entirely non- basic, tolerating the presence of free alcohols and
Despite this high reactivity, organocerium reagents are almost entirely non- basic, tolerating the presence of free alcohols and 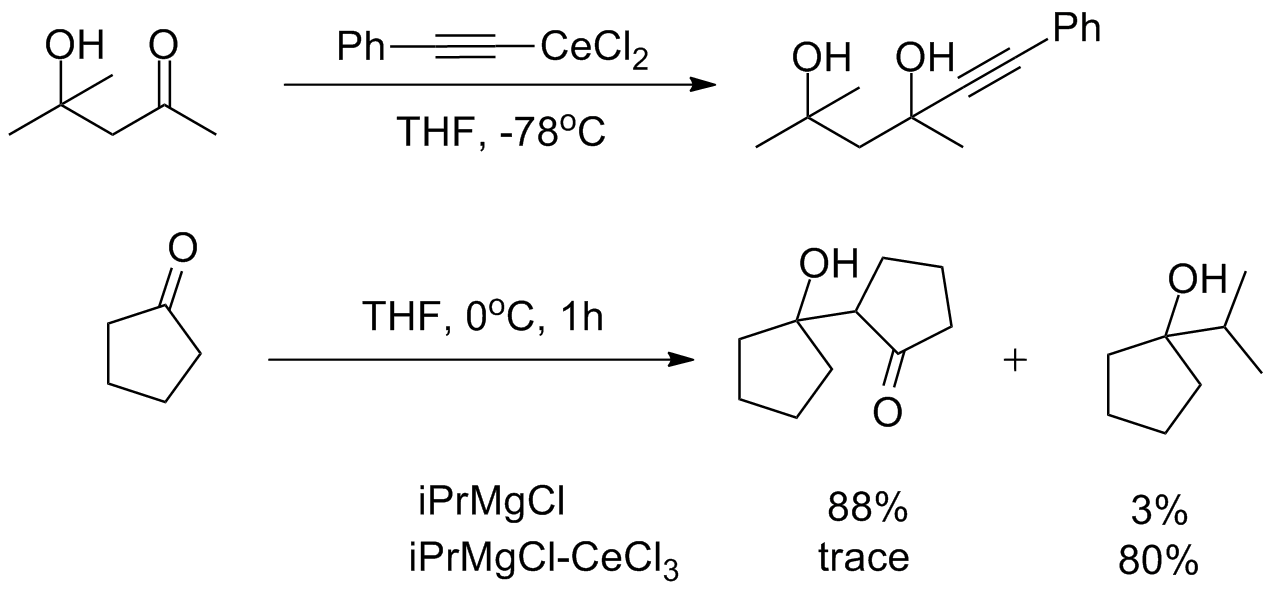 The
The  Finally, organocerium reagents have been employed in a number of total syntheses. Shown below is a key coupling step in the total synthesis of
Finally, organocerium reagents have been employed in a number of total syntheses. Shown below is a key coupling step in the total synthesis of 
 Organocerium chemistry is the science exploring the properties, structure, and reactivity of organocerium compounds,
Organocerium chemistry is the science exploring the properties, structure, and reactivity of organocerium compounds, chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
s that contain one or more chemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of ...
between carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon mak ...
and cerium
Cerium is a chemical element with the symbol Ce and atomic number 58. Cerium is a soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it often shows the +3 ...
. These compounds comprise a subset of the organolanthanides. In general, organocerium compounds are not isolable, and are rather studied in solution via their reactions with other species. There are notable exceptions, such as the Cp*3Ce(III) complex shown at right, but they are relatively rare. Complexes involving cerium of various oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
s are known: though lanthanides are most stable in the +3 state, complex
Complex commonly refers to:
* Complexity, the behaviour of a system whose components interact in multiple ways so possible interactions are difficult to describe
** Complex system, a system composed of many components which may interact with each ...
es of cerium(IV) have been reported. These latter compounds have found less widespread use due to their oxidizing nature, and the majority of literature regarding organometallic cerium complexes involves the +3 oxidation state. In particular, organocerium compounds have been developed extensively as non- basic carbon nucleophiles in organic synthesis. Because cerium is relatively non-toxic, they serve as an "environmentally friendly" alternative to other organometallic reagents. Several reviews detailing these applications have been published.
Structure
The solution structure of organocerium reagents remains unclear, although there is agreement that it depends heavily on the manner in which it is prepared. In particular, those derived from organolithium reagents likely are believed to form something similar to a 'true' organocerium structure, "R-CeCl2," while those derived from Grignard reagents are more appropriately characterized as -ate complexes of the form "R-MgX•CeCl3". Furthermore, the solvent seems to alter the solution structure of the complex, with differences noted between reagents prepared indiethyl ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable li ...
and tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
. There is evidence that the parent chloride forms a polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
ic species in THF solution, of the form μ-Cl)2(H2O)(THF)2.html" ;"title="bridging_ligand.html" ;"title="e(bridging ligand">μ-Cl)2(H2O)(THF)2">bridging_ligand.html" ;"title="e(bridging ligand">μ-Cl)2(H2O)(THF)2sub>n, but whether this type of polymer exists once the organometallic reagent is formed is unknown.
Preparation
Organocerium compounds are typically prepared via transmetallation from the respective organolithium or Grignard reagent. The most common cerium source for this purpose is cerium (III) chloride, which can be obtained in anhydrous form via dehydration (chemistry), dehydration of the commercially available hepta hydrate. Precomplexation withtetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
is important for the success of the transmetallation, with most procedures involving "vigorous stirring for a period of no less than 2 hours".
Reagents derived from alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloa ...
, alkynyl
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and ...
, and alkenyl
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, a ...
organometallic reagents as well as cerium enolate
In organic chemistry, enolates are organic anions derived from the deprotonation of carbonyl () compounds. Rarely isolated, they are widely used as reagents in the synthesis of organic compounds.
Bonding and structure
Enolate anions are electr ...
s have been described. The stability of each is approximately the same regardless of origin (i.e. lithiate or Grignard), with the exception of alkenyl reagents, which tend to be more stable when derived from the corresponding lithiate. The reasons for this are still poorly understood. Functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the re ...
s compatible with the parent organometallic compound are generally also stable upon transmetallation to cerium. The figure below summarizes the kinds of organocerium compounds that have been prepared.

Reactions
Organocerium reagents are used almost exclusively foraddition reaction
In organic chemistry, an addition reaction is, in simplest terms, an organic reaction where two or more molecules combine to form a larger one (the adduct)..
Addition reactions are limited to chemical compounds that have multiple bonds, such as ...
s in the same vein as organolithium and Grignard reagents. To this end, they have a number of particularly useful characteristics that distinguish them from their more common counterparts.
They are incredibly nucleophilic
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
, allowing additions to imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bon ...
s in the absence of additional Lewis acid catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
s, making them useful for substrates in which typical conditions fail.
 Despite this high reactivity, organocerium reagents are almost entirely non- basic, tolerating the presence of free alcohols and
Despite this high reactivity, organocerium reagents are almost entirely non- basic, tolerating the presence of free alcohols and amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen
Hydrogen is the chemical element wi ...
s as well as enol
In organic chemistry, alkenols (shortened to enols) are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene ( olefin) with a hydroxyl group attached to one end of the alkene double bond (). The t ...
izable α-protons.
 The
The oxophilicity
Oxophilicity is the tendency of certain chemical compounds to form oxides by hydrolysis or abstraction of an oxygen atom from another molecule, often from organic compounds. The term is often used to describe metal centers, commonly the early trans ...
of cerium imparts strong 1,2-selectivity in reactions with conjugated electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carrie ...
s similarly to organolithium reagents. At the same time, organocerium reagents can be used to synthesize ketones from acyl
In chemistry, an acyl group is a moiety derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids. It contains a double-bonded oxygen atom and an alkyl group (). In organic chemistry, the acyl group (IUPAC ...
compounds without over-addition, as seen with organocuprates. This dichotomy illustrates the unique reactivity of organolanthanide reagents.
 Finally, organocerium reagents have been employed in a number of total syntheses. Shown below is a key coupling step in the total synthesis of
Finally, organocerium reagents have been employed in a number of total syntheses. Shown below is a key coupling step in the total synthesis of roseophilin
Roseophilin is an antibiotic isolated from '' Streptomyces griseoviridis'' shown to have antitumor activity. The chemical structure can be considered in terms of two components, a macrotricyclic segment and a heterocyclic side-chain. Several la ...
, a potent antitumor
Cancer can be treated by surgery, chemotherapy, radiation therapy, hormonal therapy, targeted therapy (including immunotherapy such as monoclonal antibody therapy) and synthetic lethality, most commonly as a series of separate treatments (e.g. ...
antibiotic.

See also
*Luche reduction
Luche reduction is the selective organic reduction of α,β-unsaturated ketones to allylic alcohols with sodium borohydride (NaBH4) and lanthanide chlorides, mainly cerium(III) chloride (CeCl3), in methanol or ethanol. The Luche reduction ca ...
* Cerium
Cerium is a chemical element with the symbol Ce and atomic number 58. Cerium is a soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it often shows the +3 ...
References
{{ChemicalBondsToCarboncerium
Cerium is a chemical element with the symbol Ce and atomic number 58. Cerium is a soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it often shows the +3 ...
cerium
Cerium is a chemical element with the symbol Ce and atomic number 58. Cerium is a soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it often shows the +3 ...
Cerium