Organoactinide on:
[Wikipedia]
[Google]
[Amazon]
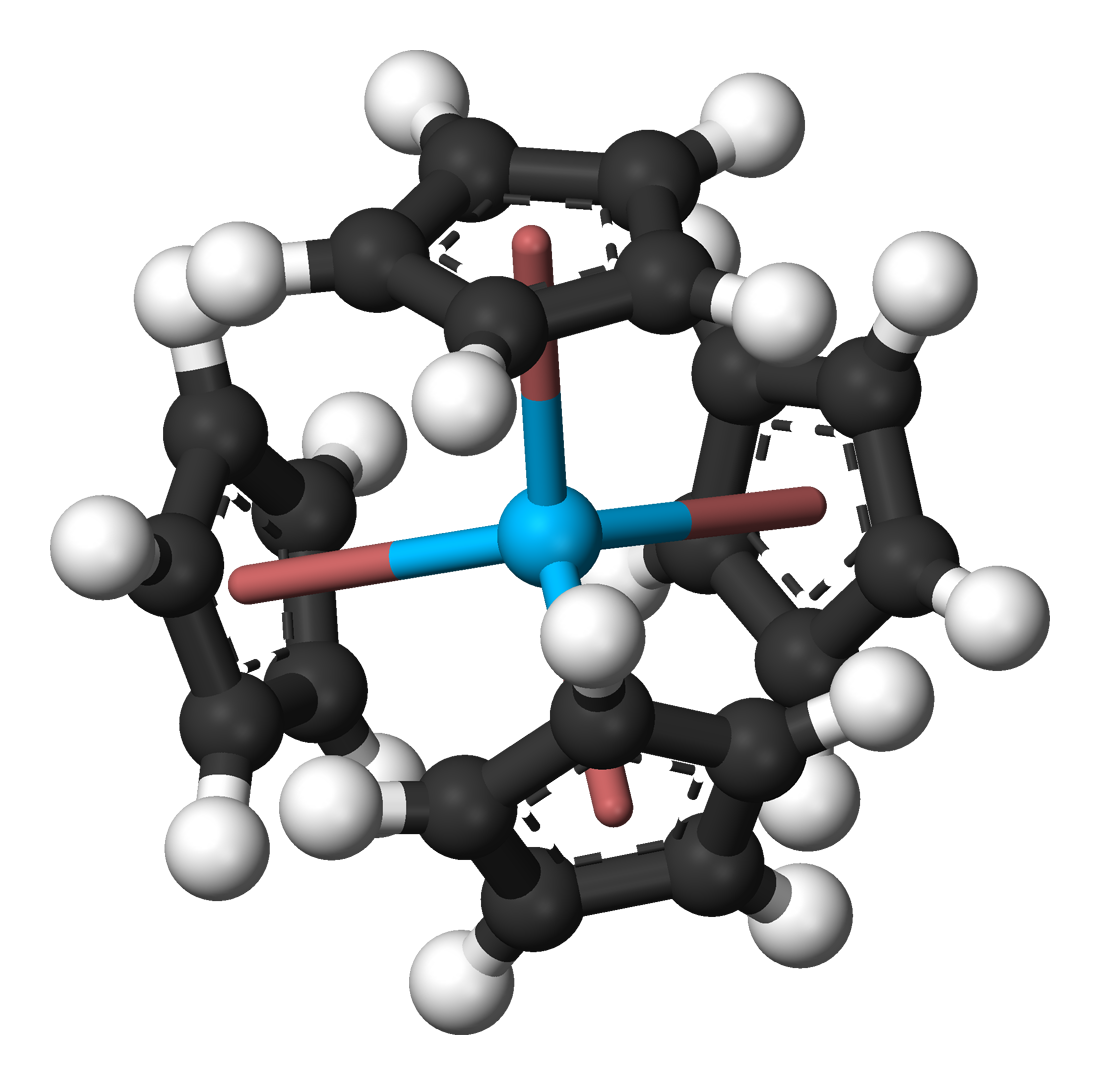 Organoactinide chemistry is the science exploring the properties, structure and reactivity of organoactinide compounds, which are
Organoactinide chemistry is the science exploring the properties, structure and reactivity of organoactinide compounds, which are
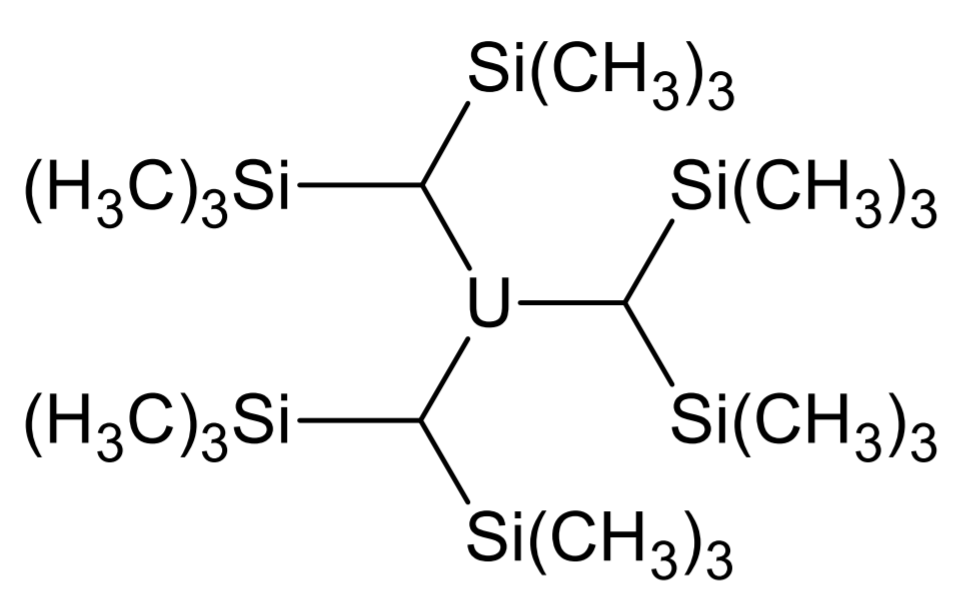 Attempts to synthesize uranium alkyls were first made during the Manhattan project by
Attempts to synthesize uranium alkyls were first made during the Manhattan project by
 Actinides form sandwich complexes with cyclooctatetraene analogously to how transition metals react with cyclopentadienyl ligands. Actinide ions have atomic radii that are too large to form MCp2 compounds, so that they prefer to react with C8H82- ions instead.
The first example of this type of chemical species was discovered in 1968 by
Actinides form sandwich complexes with cyclooctatetraene analogously to how transition metals react with cyclopentadienyl ligands. Actinide ions have atomic radii that are too large to form MCp2 compounds, so that they prefer to react with C8H82- ions instead.
The first example of this type of chemical species was discovered in 1968 by  Most tetravalent actinides react similarly to form actinocenes:
Bis(cyclooctatetraene)protactinium was first prepared in 1973 by turning
Most tetravalent actinides react similarly to form actinocenes:
Bis(cyclooctatetraene)protactinium was first prepared in 1973 by turning Pa2O5 + SOCl2 -> 00CPaCl5
:3PaCl5 + Al -> 3PaCl4 + AlCl3
:PaCl4 + 2K2(COT) -> Pa(COT)2 + 4KCl :
AmI3 + K2(COT) -> KAm(COT)2
This compound is present in solution as the THF adduct.
 Plutonium also forms a sandwich complex with 1,4-bis(trimethylsilyl)cyclooctatetraenyl (1,4-COT’’) and its 1,3 isomer. This compound is prepared by the oxidation of the anionic green Pu(III) complex Li(THF)4 u(1,4-COT’’)2with
Plutonium also forms a sandwich complex with 1,4-bis(trimethylsilyl)cyclooctatetraenyl (1,4-COT’’) and its 1,3 isomer. This compound is prepared by the oxidation of the anionic green Pu(III) complex Li(THF)4 u(1,4-COT’’)2with  The neptunium equivalent with the trisubstituted COT’’’ has also been reported and the complexes of both the tri- and di- substituted ligands with thorium and uranium are well known. They were synthesized according to the following reaction schemes:
The neptunium equivalent with the trisubstituted COT’’’ has also been reported and the complexes of both the tri- and di- substituted ligands with thorium and uranium are well known. They were synthesized according to the following reaction schemes:

 Most trivalent f block elements form compounds with cyclopentadiene with the formula . These complexes have been isolated up to
Most trivalent f block elements form compounds with cyclopentadiene with the formula . These complexes have been isolated up to  The synthesis of the AnCp3 usually follows the reaction scheme shown above with a few more added steps that are sometimes needed to synthesize the trichlorides from the commercially supplied oxides. Nevertheless, other syntheses are also used by some authors: alkali metal cyclopentadienides can be used instead of the beryllium complex, and An(IV) complexes can also be used via a
The synthesis of the AnCp3 usually follows the reaction scheme shown above with a few more added steps that are sometimes needed to synthesize the trichlorides from the commercially supplied oxides. Nevertheless, other syntheses are also used by some authors: alkali metal cyclopentadienides can be used instead of the beryllium complex, and An(IV) complexes can also be used via a

 Organoactinide chemistry is the science exploring the properties, structure and reactivity of organoactinide compounds, which are
Organoactinide chemistry is the science exploring the properties, structure and reactivity of organoactinide compounds, which are organometallic compound
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and s ...
s containing a carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon mak ...
to actinide
The actinide () or actinoid () series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The info ...
chemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of ...
.
Like most organometallic compounds, the organoactinides are air sensitive and need to be handled using the appropriate methods.
Organometallic complexes with σ-bonding
Most common organoactinide complexes involve π-bonding with ligands such as cyclopentadienyl, but there are a few exceptions with σ-bonding, namely inthorium
Thorium is a weakly radioactive metallic chemical element with the symbol Th and atomic number 90. Thorium is silvery and tarnishes black when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high ...
and uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
chemistry as these are the most easily handleable elements of this group.
Alkyl and aryl compounds
 Attempts to synthesize uranium alkyls were first made during the Manhattan project by
Attempts to synthesize uranium alkyls were first made during the Manhattan project by Henry Gilman
Henry Gilman (May 9, 1893 – November 7, 1986) was an American organic chemist known as the father of organometallic chemistry, the field within which his most notable work was done. He discovered the Gilman reagent, which bears his name.
Earl ...
, inspired by the volatility of main group organometallics. However he noticed that these compounds tend to be highly unstable.
Marks and Seyam attempted to synthesize them from UCl using organolithium reagent
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
s, but these decomposed quickly.
In 1989, a group finally synthesized a homoleptic
In inorganic chemistry, a homoleptic chemical compound is a metal compound with all ligands identical. The term uses the " homo-" prefix to indicate that something is the same for all. Any metal species which has more than one type of ligand is he ...
complex
Complex commonly refers to:
* Complexity, the behaviour of a system whose components interact in multiple ways so possible interactions are difficult to describe
** Complex system, a system composed of many components which may interact with each ...
with trimethylsilyl
A trimethylsilyl group (abbreviated TMS) is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom minus;Si(CH3)3 which is in turn bonded to the rest of a molecule. This structural group is ch ...
groups: . Since then, variants of higher coordination number
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it. The ion/molecule/atom surrounding the central io ...
s such as have also been synthesized.
On the other hand, only one homoleptic thorium alkyl is known. The seven coordinate heptamethylthorate(IV) anion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
was synthesized in 1984 using a similar procedure to the equivalent uranium complex.
Mixed phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
containing complexes of thorium and uranium tetramethyls have also been made, using dmpe as the organophosphorus ligand stabilising the structure (amides can also assume this role).
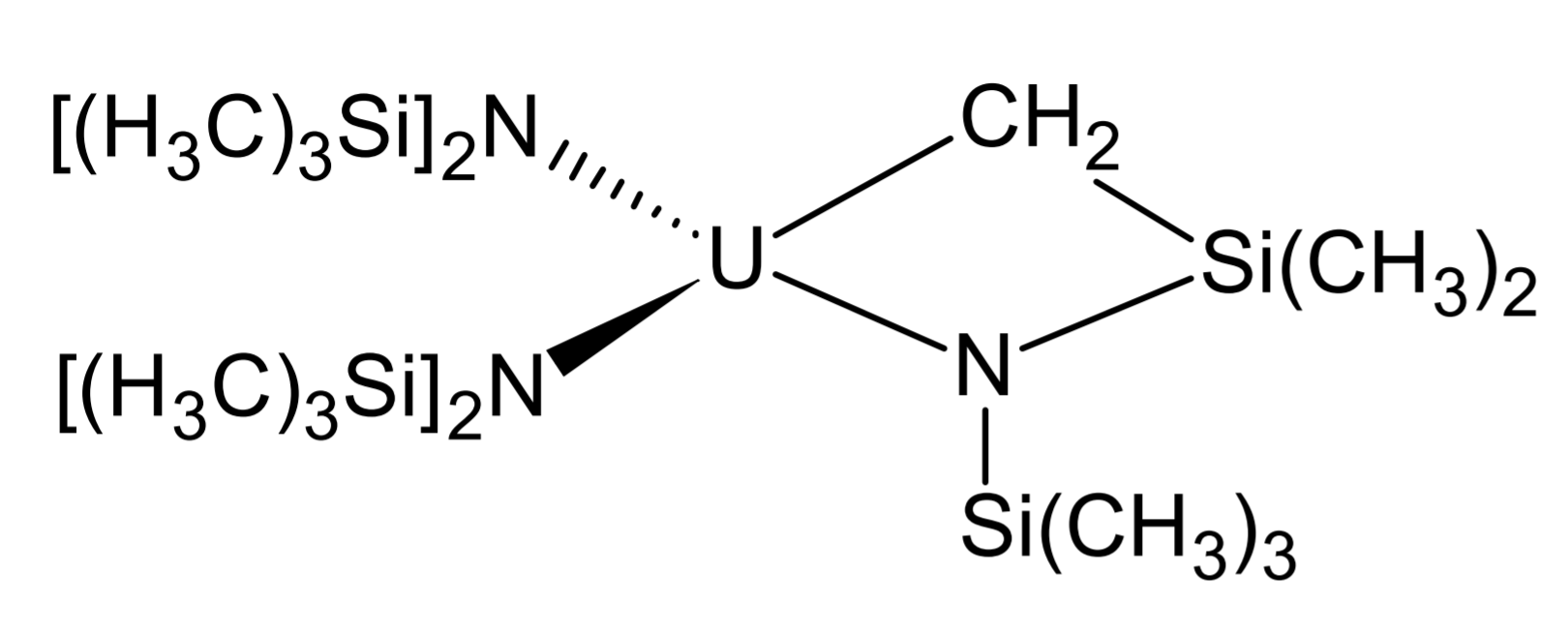
Metallacycles
Uranium and thorium both form metallacycles with a diverse chemistry. These complexes are very labile so trimethylsilyl groups are again present for protection. These compounds are formed by reacting weaker alkylating agents ( and are too strong and lead to the formation of simple alkyls) with (An = Th, U).
Organometallic complexes with π-bonding
A large majority of the organoactinides involve Cyclopentadienyl (Cp) orCyclooctatetraene
1,3,5,7-Cyclooctatetraene (COT) is an unsaturated derivative of cyclooctane, with the formula C8H8. It is also known as nnulene. This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature. Because of ...
(COT) and their derivatives as ligands. These usually take part in η5- and η8-bonding, donating electron density through their pi orbitals.
Cyclooctatetraene complexes
Actinocenes
 Actinides form sandwich complexes with cyclooctatetraene analogously to how transition metals react with cyclopentadienyl ligands. Actinide ions have atomic radii that are too large to form MCp2 compounds, so that they prefer to react with C8H82- ions instead.
The first example of this type of chemical species was discovered in 1968 by
Actinides form sandwich complexes with cyclooctatetraene analogously to how transition metals react with cyclopentadienyl ligands. Actinide ions have atomic radii that are too large to form MCp2 compounds, so that they prefer to react with C8H82- ions instead.
The first example of this type of chemical species was discovered in 1968 by Andrew Streitwieser
Andrew Streitwieser was an American chemist known for his contributions to physical organic chemistry.
Streitwieser was born in 1927 in Buffalo, New York and he grew up in New York City. He attended Columbia College and then Columbia Univers ...
, who prepared uranocene
Uranocene, U(C8H8)2, is an organouranium compound composed of a uranium atom sandwiched between two cyclooctatetraenide rings. It was one of the first organoactinide compounds to be synthesized. It is a green air-sensitive solid that dissolves in ...
by reacting with in tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
at 0 °C. The compound itself is a pyrophoric
A substance is pyrophoric (from grc-gre, πυροφόρος, , 'fire-bearing') if it ignites spontaneously in air at or below (for gases) or within 5 minutes after coming into contact with air (for liquids and solids). Examples are organolit ...
green solid that is otherwise quite unreactive.
protactinium(V) oxide
Protactinium(V) oxide is a chemical compound with the formula Pa2 O5. When it is reduced with hydrogen, it forms PaO2. Aristid V. Grosse was first to prepare 2 mg of Pa2O5 in 1927. Pa2O5 does not dissolve in concentrated HNO3, but dissolve ...
into the pentachloride and reducing it with aluminium powder before reacting it with potassium cyclooctatetraenide
Dipotassium cyclooctatetraenide, sometimes abbreviated K2COT, is an organopotassium compound with the formula K2C8H8. It is a brown solid that is used as a precursor to cyclooctatetraenide complexes, such as uranocene (U(C8H8)2). Analogs of K2 ...
.
:Neptunocene
Neptunocene, Np(C8H8)2, is an organoneptunium compound composed of a neptunium atom sandwiched between two cyclooctatetraenide (COT2-) rings. As a solid it has a dark brown/red colour but it appears yellow when dissolved in chlorocarbons, in whi ...
and thorocene were made similarly using the tetrachlorides. Plutonocene is the exception here: as there is no stable plutonium(IV) chloride known, (Hpy)2PuCl6 had to be used.
The later actinides also form complexes with COT but these don't usually assume the classic neutral sandwich structure. Trivalent actinides form ionic compounds with COT ligands, this can be exemplified by the reaction of americium triiodide with K2COT.
:Complexes of substituted cyclooctatetraenes
Many substituted uranocenes have been synthesized. The methodology followed was the same as for simple , but the properties of some of the compounds were found to be different. The tetraphenylcyclooctatetraene complex was found to be completely air stable by Streitwieser. This high stability is probably due to the hindering effects of the phenyl groups, protecting the U4+ center from an attack byoxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
.
All these derivatives are much more soluble in organic solvents such as benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms ...
, in which they form green solutions that are more air sensitive than the crystalline solids.
 Plutonium also forms a sandwich complex with 1,4-bis(trimethylsilyl)cyclooctatetraenyl (1,4-COT’’) and its 1,3 isomer. This compound is prepared by the oxidation of the anionic green Pu(III) complex Li(THF)4 u(1,4-COT’’)2with
Plutonium also forms a sandwich complex with 1,4-bis(trimethylsilyl)cyclooctatetraenyl (1,4-COT’’) and its 1,3 isomer. This compound is prepared by the oxidation of the anionic green Pu(III) complex Li(THF)4 u(1,4-COT’’)2with cobalt(II) chloride
Cobalt(II) chloride is an inorganic compound of cobalt and chlorine, with the formula . The compound forms several hydrates ·''n'', for ''n'' = 1, 2, 6, and 9. Claims of the formation of tri- and tetrahydrates have not been confirmed.M. T. Saug ...
which leads to the formation of Pu(1,4-COT’’)(1,3-COT’’). The reaction is easily noticeable by the THF solution changing to a dark red colour, characteristic of Pu(IV).
 The neptunium equivalent with the trisubstituted COT’’’ has also been reported and the complexes of both the tri- and di- substituted ligands with thorium and uranium are well known. They were synthesized according to the following reaction schemes:
The neptunium equivalent with the trisubstituted COT’’’ has also been reported and the complexes of both the tri- and di- substituted ligands with thorium and uranium are well known. They were synthesized according to the following reaction schemes:

Cyclopentadiene complexes
Tris(cyclopentadienyl)actinide complexes
 Most trivalent f block elements form compounds with cyclopentadiene with the formula . These complexes have been isolated up to
Most trivalent f block elements form compounds with cyclopentadiene with the formula . These complexes have been isolated up to californium
Californium is a radioactive chemical element with the symbol Cf and atomic number 98. The element was first synthesized in 1950 at Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory), by bombarding ...
, with the einsteinium
Einsteinium is a synthetic element with the symbol Es and atomic number 99. Einsteinium is a member of the actinide series and it is the seventh transuranium element. It was named in honor of Albert Einstein.
Einsteinium was discovered as a com ...
equivalent having been observed in the gas phase.
 The synthesis of the AnCp3 usually follows the reaction scheme shown above with a few more added steps that are sometimes needed to synthesize the trichlorides from the commercially supplied oxides. Nevertheless, other syntheses are also used by some authors: alkali metal cyclopentadienides can be used instead of the beryllium complex, and An(IV) complexes can also be used via a
The synthesis of the AnCp3 usually follows the reaction scheme shown above with a few more added steps that are sometimes needed to synthesize the trichlorides from the commercially supplied oxides. Nevertheless, other syntheses are also used by some authors: alkali metal cyclopentadienides can be used instead of the beryllium complex, and An(IV) complexes can also be used via a reductive elimination
Reductive elimination is an elementary step in organometallic chemistry in which the oxidation state of the metal center decreases while forming a new covalent bond between two ligands. It is the microscopic reverse of oxidative addition, and is ...
reaction.
These compounds have been known since the sixties, however until 2018 only the neptunium compound was structurally characterised. Kovàcs and coworkers were able to analyse the plutonium and uranium complexes, finding that all three structures were similar, with an asymmetrical distribution of cylopentadienide ligands and a higher covalent character to the carbon-actinide bond than in organolanthanide compounds.
Tetrakis(cyclopentadienyl)actinide complexes
Tetravalent thorium, uranium and neptunium easily form MCp4 compounds by a metathesis reaction from potassium cyclopentadienide using benzene as a solvent.
See also
* Organouranium chemistry * Organoneptunium chemistry *Actinocenes
Actinocenes are a family of organoactinide compounds consisting of metallocenes containing elements from the actinide series. They typically have a sandwich structure with two dianionic cyclooctatetraenyl ligands (COT2-, which is ) bound to an a ...
**Uranocene
Uranocene, U(C8H8)2, is an organouranium compound composed of a uranium atom sandwiched between two cyclooctatetraenide rings. It was one of the first organoactinide compounds to be synthesized. It is a green air-sensitive solid that dissolves in ...
, U(C8H8)2
**Neptunocene
Neptunocene, Np(C8H8)2, is an organoneptunium compound composed of a neptunium atom sandwiched between two cyclooctatetraenide (COT2-) rings. As a solid it has a dark brown/red colour but it appears yellow when dissolved in chlorocarbons, in whi ...
, Np(C8H8)2
**Plutonocene
Plutonocene, Pu(C8H8)2, is an organoplutonium compound composed of a plutonium atom sandwiched between two cyclooctatetraenide (COT2-) rings. It is a dark red, very air-sensitive solid that is sparingly soluble in toluene and chlorocarbons. Plut ...
, Pu(C8H8)2
References
* {{ChemicalBondsToCarbon