Oilfield Scale Inhibition on:
[Wikipedia]
[Google]
[Amazon]
Oilfield scale inhibition is the process of preventing the formation of scale from blocking or hindering fluid flow through pipelines, valves, and pumps used in oil production and processing. Scale inhibitors (SIs) are a class of specialty chemicals that are used to slow or prevent scaling in water systems. Oilfield scaling is the
 The three prevailing water-related problems that upset oil companies today are corrosion, gas hydrates and scaling in production systems. The reservoir water has a high composition of dissolved minerals equilibrated over millions of years at constant physicochemical conditions. As the reservoir fluids are pumped from the ground, changes in temperature, pressure and chemical composition shift the equilibria and cause precipitation and deposition of sparingly soluble salts that build up over time with the potential of blocking vital assets in the oil production setups. Scaling can occur at all stages of oil/gas production systems (upstream, midstream and downstream) and causes blockages of well-bore perforations, casing, pipelines, pumps, valves etc. Severe scaling issues have been reported in Russia and certain North Sea production systems.
The three prevailing water-related problems that upset oil companies today are corrosion, gas hydrates and scaling in production systems. The reservoir water has a high composition of dissolved minerals equilibrated over millions of years at constant physicochemical conditions. As the reservoir fluids are pumped from the ground, changes in temperature, pressure and chemical composition shift the equilibria and cause precipitation and deposition of sparingly soluble salts that build up over time with the potential of blocking vital assets in the oil production setups. Scaling can occur at all stages of oil/gas production systems (upstream, midstream and downstream) and causes blockages of well-bore perforations, casing, pipelines, pumps, valves etc. Severe scaling issues have been reported in Russia and certain North Sea production systems.
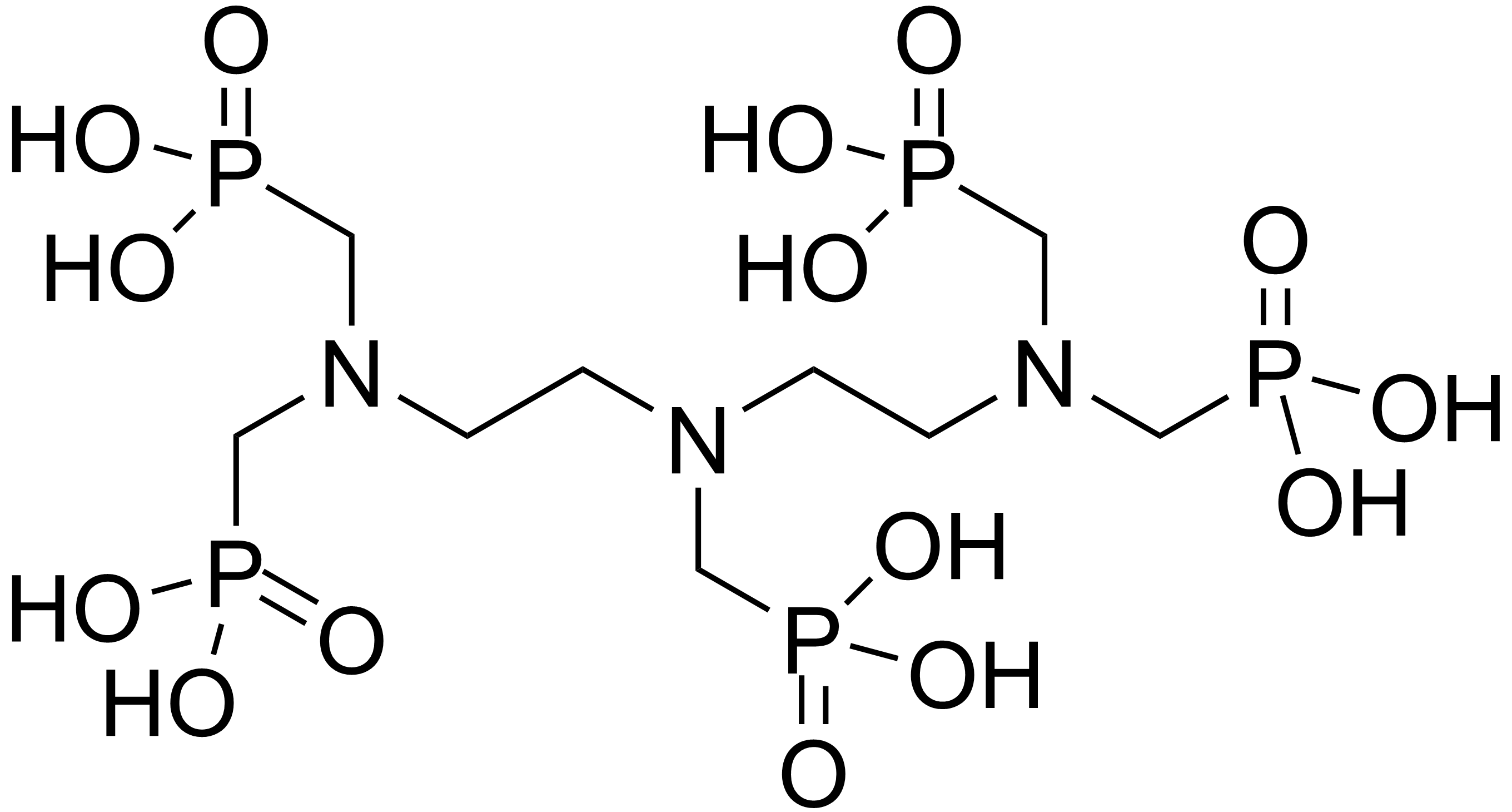 Scale inhibitors are specialty chemicals that are added to oil production systems to delay, reduce and/or prevent scale deposition.
Scale inhibitors are specialty chemicals that are added to oil production systems to delay, reduce and/or prevent scale deposition.
precipitation
In meteorology, precipitation is any product of the condensation of atmospheric water vapor that falls under gravitational pull from clouds. The main forms of precipitation include drizzle, rain, sleet, snow, ice pellets, graupel and hail. ...
and accumulation of insoluble crystals (salts) from a mixture of incompatible aqueous
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, or sodium chloride (NaCl), in water would be rep ...
phases in oil processing systems. is a common term in the oil industry
The petroleum industry, also known as the oil industry or the oil patch, includes the global processes of exploration, extraction, refining, transportation (often by oil tankers and pipelines), and marketing of petroleum products. The larges ...
used to describe solid deposits that grow over time, blocking and hindering fluid flow through pipelines, valves, pumps etc. with significant reduction in production rates and equipment damages. Scaling represents a major challenge for flow assurance Flow assurance is a relatively new term in oil and gas industry. It refers to ensuring successful and economical flow of hydrocarbon stream from reservoir to the point of sale. The term was coined by Petrobras in the early 1990s ahead of a DeepStar ...
in the oil and gas industry. Examples of oilfield scales are calcium carbonate (limescale
Limescale is a hard, chalky deposit, consisting mainly of calcium carbonate (CaCO3). It often builds up inside kettles, boilers, and pipework, especially that for hot water. It is also often found as a similar deposit on the inner surfaces of old ...
), iron sulfides, barium sulfate and strontium sulfate. Scale inhibition encompasses the processes or techniques employed to treat scaling problems.
Background
 The three prevailing water-related problems that upset oil companies today are corrosion, gas hydrates and scaling in production systems. The reservoir water has a high composition of dissolved minerals equilibrated over millions of years at constant physicochemical conditions. As the reservoir fluids are pumped from the ground, changes in temperature, pressure and chemical composition shift the equilibria and cause precipitation and deposition of sparingly soluble salts that build up over time with the potential of blocking vital assets in the oil production setups. Scaling can occur at all stages of oil/gas production systems (upstream, midstream and downstream) and causes blockages of well-bore perforations, casing, pipelines, pumps, valves etc. Severe scaling issues have been reported in Russia and certain North Sea production systems.
The three prevailing water-related problems that upset oil companies today are corrosion, gas hydrates and scaling in production systems. The reservoir water has a high composition of dissolved minerals equilibrated over millions of years at constant physicochemical conditions. As the reservoir fluids are pumped from the ground, changes in temperature, pressure and chemical composition shift the equilibria and cause precipitation and deposition of sparingly soluble salts that build up over time with the potential of blocking vital assets in the oil production setups. Scaling can occur at all stages of oil/gas production systems (upstream, midstream and downstream) and causes blockages of well-bore perforations, casing, pipelines, pumps, valves etc. Severe scaling issues have been reported in Russia and certain North Sea production systems.
Types of scales
Two main classifications of scales are known; inorganic and organic scales and the two types are mutually inclusive, occurring simultaneously in the same system, referred to as mixed scale. Mixed scales may result in highly complex structured scales that are difficult to treat. Such scales require aggressive, severe and sometimes costly remediation techniques.Paraffin wax
Paraffin wax (or petroleum wax) is a soft colorless solid derived from petroleum, coal, or oil shale that consists of a mixture of hydrocarbon molecules containing between 20 and 40 carbon atoms. It is solid at room temperature and begins to m ...
, asphaltene
Asphaltenes are molecular substances that are found in crude oil, along with resins, aromatic hydrocarbons, and saturates (i.e. saturated hydrocarbons such as alkanes). The word "asphaltene" was coined by Boussingault in 1837 when he noticed tha ...
s and gas hydrates
Clathrate hydrates, or gas hydrates, clathrates, hydrates, etc., are crystalline water-based solids physically resembling ice, in which small non-polar molecules (typically gases) or polar molecules with large hydrophobic moieties are trapped ins ...
are the most often encountered organic scales in the oil industry. This article focuses on the simplest and common form of scales encountered; inorganic scales.
Inorganic scale
Inorganic scales refer tomineral deposits
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid chemical compound with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. (2 ...
that occur when the formation water mixes with different brine
Brine is a high-concentration solution of salt (NaCl) in water (H2O). In diverse contexts, ''brine'' may refer to the salt solutions ranging from about 3.5% (a typical concentration of seawater, on the lower end of that of solutions used for br ...
s such as injection water. The mixing changes causes reaction between incompatible ions and changes the thermodynamic and equilibrium state of the reservoir fluids. Supersaturation
In physical chemistry, supersaturation occurs with a solution when the concentration of a solute exceeds the concentration specified by the value of solubility at equilibrium. Most commonly the term is applied to a solution of a solid in a liqu ...
and subsequent deposition of the inorganic salts occur. The most common types of inorganic scales known to the oil/gas industry are carbonates
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonate g ...
and sulfates
The sulfate or sulphate ion is a polyatomic
A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge t ...
; sulfides
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to chemical compounds la ...
and chlorites
The chlorite ion, or chlorine dioxide anion, is the halite with the chemical formula of . A chlorite (compound) is a compound that contains this group, with chlorine in the oxidation state of +3. Chlorites are also known as salts of chlorous ac ...
are often encountered.
While the solubility of most inorganic salts (NaCl, KCl, ...) increases with temperature (endothermic dissolution reaction), some inorganic salts such as calcium carbonate and calcium sulfate have also a retrograde solubility
Retrograde may refer to:
Film and television
* ''Retrograde'' (2004 film), a film by Christopher Kulikowski
* ''Retrograde'' (2022 film), a documentary film by Matthew Heineman
* ''Retrograde'' (TV series), a 2020 Australian television comedy ...
, i.e., their solubility decreases with temperature. In the case of calcium carbonate, it is due to the degassing of CO2 whose solubility decreases with temperature as is the case for most of the gases (exothermic dissolution reaction in water). In calcium sulfate, the reason is that the dissolution reaction of calcium sulfate itself is exothermic and therefore is favored when the temperature decreases (then, the dissolution heat is more easily evacuated; see Le Chatelier's principle
Le Chatelier's principle (pronounced or ), also called Chatelier's principle (or the Equilibrium Law), is a principle of chemistry used to predict the effect of a change in conditions on chemical equilibria. The principle is named after French c ...
). In other terms, the solubility of calcium carbonate and calcium sulfate increases at low temperature and decreases at high temperature, as it is also the case for calcium hydroxide
Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca( OH)2. It is a colorless crystal or white powder and is produced when quicklime (calcium oxide) is mixed or slaked with water. It has m ...
(portlandite
Portlandite is a hydroxide-bearing mineral typically included in the oxide mineral class. It is the naturally occurring form of calcium hydroxide (Ca(OH)2) and the calcium analogue of brucite (Mg(OH)2).
Occurrence
Portlandite occurs in a variety ...
), often cited as a didactic case study to explain the reason of retrograde solubility.
Calcium carbonate scale
Water, noted for its high solvation power can dissolve certain gases such as carbon dioxide (CO2) to form aqueous CO2(aq). Under the right conditions of temperature and/or pressure, H2O and CO2(aq) molecules react to yield carbonic acid (H2CO3) whose solubility increases at low temperature and high pressure. The slightest changes in pressure and temperature dissolves H2CO3(aq) in water according to equation (3) to form hydronium and bicarbonate (HCO3−(aq)) ions. # CO2(aq) + H2O(l) ↔ H2CO3(aq) # H2CO3(aq) ↔ H+(aq) + HCO3−(aq) # 2 HCO3−(aq) ↔ CO32−(aq) + H2O(l) + CO2(g) # Ca2+(aq) + CO32−(aq) ↔ CaCO3(s) The two reactions (2) and (4) describe the equilibrium between bicarbonate ions (HCO3−), which are highly soluble in water and calcium carbonate (CaCO3) salt. According toLe Chatelier's principle
Le Chatelier's principle (pronounced or ), also called Chatelier's principle (or the Equilibrium Law), is a principle of chemistry used to predict the effect of a change in conditions on chemical equilibria. The principle is named after French c ...
, drilling operations and extraction of the oil from the well bore decreases the pressure of the formation and the equilibrium shifts to the right (3) to increase the production of CO2 to offset the change in pressure. After years of oil production, wells may experience significant pressure drops resulting in large CaCO3 deposits as the equilibrium shifts to offset the pressure changes.
Sulfate scales
Sulfates of Group (II) metal ions (M2+), generally decrease in solubility down the group. The most difficult scales to remove are those of Barium sulfate because of its high insolubility forming very hard scale deposits. A general representation of the reaction is summarized in reaction: 5. M2+(aq) + SO42−(aq) → MSO4(s) Sulfate scale usually forms when formation water and injected seawater mix together. The relationship between these and the degree of supersaturation is crucial in estimating the amount of sulfate salts that will precipitate in the system. Seawater has a high concentration of sulfate ions and mixing with formation water with many Ca2+ and other M2+ ions in the formation water. Severe problems with sulfate scale are common in reservoirs where seawater has been injected to enhance oil recovery. Due to its relatively high solubility in water, Calcium sulfate is the easiest sulfate scale to remove chemically as compared to strontium and barium sulfate. Scale crystals are initially dispersed in production systems until accumulation of stable crystals of insoluble sulfates and scale growth occur at nucleation centers. Uneven pipeline surfaces and production equipment such as pumps and valves cause rapid scale growth to levels that can block pipelines. The scaling-tendency of an oil-well can be predicted based on the prevailing conditions such as pH, temperature, pressure, ionic strength and the mole fraction of CO2 in the vapor and aqueous phases. For instance the saturation index for CaCO3 scale is calculated using the formula; Fs= /Ksp Where Fs is the scale saturation ratio, defined as the ratio of the activity product to the solubility product of the salt. Activity is defined as the product of the activity coefficients and the concentrations of Ca2+ and SO42− ions. The ionic strength is a measure of the concentration of the dissociated ions dissolved in water also called as “total dissolved solids” (TDS).Scale remediation
Different oilfield scale remediation techniques are known but majority are based on three basic themes: # Sulfate ion sequestering from sea injection waters # Chemical or mechanical Scale removal/dissolution # Application of Scale Inhibitors (SIs) for scale prevention The first two methods may be used for short-term treatment and effective for mild-scaling conditions, however, continuous injection or chemical scale squeeze treatment with SIs have been proven over the years to be the most efficient and cost-effective preventative technique.Scale inhibitors
 Scale inhibitors are specialty chemicals that are added to oil production systems to delay, reduce and/or prevent scale deposition.
Scale inhibitors are specialty chemicals that are added to oil production systems to delay, reduce and/or prevent scale deposition. acrylic acid
Acrylic acid (IUPAC: propenoic acid) is an organic compound with the formula CH2=CHCOOH. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has a ...
polymers, maleic acid
Maleic acid or ''cis''-butenedioic acid is an organic compound that is a dicarboxylic acid, a molecule with two carboxyl groups. Its chemical formula is HO2CCH=CHCO2H. Maleic acid is the ''cis''-isomer of butenedioic acid, whereas fumaric acid ...
polymers and phosphonate
In organic chemistry, phosphonates or phosphonic acids are organophosphorus compounds containing groups (where R = alkyl, aryl, or just hydrogen). Phosphonic acids, typically handled as salts, are generally nonvolatile solids that are poorly ...
s have been used extensively for scale treatment in water systems due to their excellent solubility, thermal stability and dosage efficiency. In the water treatment industry, the major classes of SIs have inorganic phosphate, organophosphorous and organic polymer backbones and common examples are PBTC (phosphonobutane-1,2,4-tricarboxylic acid), ATMP
ATMP or aminotris(methylenephosphonic acid) is a phosphonic acid with chemical formula N(CH2PO3H2)3. It has chelating properties. It can be synthesized from the Mannich-type reaction of ammonia, formaldehyde, and phosphorous acid, in a manner s ...
(amino-trimethylene phosphonic acid) and HEDP (1-hydroxyethylidene-1,1-diphosphonic acid), polyacrylic acid
Poly(acrylic acid) (PAA; trade name Carbomer) is a polymer with the formula (CH2-CHCO2H)n. It is a derivative of acrylic acid (CH2=CHCO2H). In addition to the homopolymers, a variety of copolymers and crosslinked polymers, and partially deproto ...
(PAA), phosphinopolyacrylates (such as PPCA), polymaleic acids (PMA), maleic acid
Maleic acid or ''cis''-butenedioic acid is an organic compound that is a dicarboxylic acid, a molecule with two carboxyl groups. Its chemical formula is HO2CCH=CHCO2H. Maleic acid is the ''cis''-isomer of butenedioic acid, whereas fumaric acid ...
terpolymers (MAT), sulfonic acid
In organic chemistry, sulfonic acid (or sulphonic acid) refers to a member of the class of organosulfur compounds with the general formula , where R is an organic alkyl or aryl group and the group a sulfonyl hydroxide. As a substituent, it is kn ...
copolymers, such as SPOCA (sulfonated phosphonocarboxylic acid), polyvinyl sulfonates. Two common oilfield mineral SIs are Poly-Phosphono Carboxylic acid (PPCA) and Diethylenetriamine- penta (methylene phosphonic acid) (DTPMP
, Section2={{Chembox Properties
, Formula=C9H28N3O15P5
, MolarMass = 573.20
, Appearance= solid
, Density=
, MeltingPt=
, BoilingPt=
, Solubility=
, Section3={{Chembox Hazards
, MainHazards=
, FlashPt=
, AutoignitionPt =
DTPMP ...
).
Inhibition of calcium carbonate scale deposition and crystal studies of its polymorphs have been conducted. Different SIs are designed for specific scaling conditions and biodegradability
Biodegradation is the breakdown of organic matter by microorganisms, such as bacteria and fungi. It is generally assumed to be a natural process, which differentiates it from composting. Composting is a human-driven process in which biodegradati ...
properties. The inhibitor molecules essentially bind ions in aqueous phase of production fluids that could potentially precipitate as scales. For instance, to bind positively charged ions in the water, anions must be present in the inhibitor molecular backbone structure and vice versa. Group (II) metal ions are commonly sequestered by SIs with the following functionalities;
- Phosphonate ions (-PO3H−)
- Phosphate ions (-OPO3H−)
- Phosphonate ions (-PO2H−)
- Sulphonate ions (-SO3−)
- Carboxylate ions (-CO2−)
A SI with a combination of two or more of these functional groups is more efficient in managing scale problems. Usually the sodium salts of the carboxylic derivatives are synthesized as the anionic derivatives and are known to be the most effective due to their high solubilities. Interactions of these functional groups tend to prevent the crystal growth sites using dissociated or un-dissociated groups. The dissociation state is determined by the pH of the system, hence knowledge of the pKa values of the chemicals are important for different pH environments. Again, the inhibition efficiency of the SI depends on its compatibility with other production chemicals such as corrosion inhibitors.
Environmental considerations
Generally, the environmental impacts of SIs are complicated further by combination of other chemicals applied through exploratory, drilling, well-completion and start-up operations. Produced fluids, and other wastes from oil and gas operations with high content of different toxic compounds are hazardous and harmful to human health, water supplies, marine and freshwater organisms. For instance trails of increased turbidity resulting from oil and gas exploratory activities on the eastern shelf of Sakhalin in Russia have been reported with consequential adverse effects on salmon, cod and littoral amphipods. Efforts to develop moreenvironmentally friendly
Environment friendly processes, or environmental-friendly processes (also referred to as eco-friendly, nature-friendly, and green), are sustainability and marketing terms referring to goods and services, laws, guidelines and policies that clai ...
SIs have been made since the late 1990s and an increasing number of such SIs are becoming commercially available. Recent environmental awareness over the past 15 years has resulted in the production and application of more environmentally friendly SIs, otherwise called 'Green Scale Inhibitors' (GSI). These GSIs are designed to have reduced bio-accumulating and high biodegradability
Biodegradation is the breakdown of organic matter by microorganisms, such as bacteria and fungi. It is generally assumed to be a natural process, which differentiates it from composting. Composting is a human-driven process in which biodegradati ...
properties and therefore reduce pollution of the waters around oil production systems. Phosphate ester
In organic chemistry, organophosphates (also known as phosphate esters, or OPEs) are a class of organophosphorus compounds with the general structure , a central phosphate molecule with alkyl or Aryl, aromatic substituents. They can be conside ...
SIs, commonly employed for treating calcium carbonate scales are known to be environmentally friendly but poor inhibition efficiency. Release of SIs containing Nitrogen and Phosphorus distorts the natural equilibrium of the immediate water body with adverse effects on aquatic life.
Another alternative, polysaccharide SIs meet the requirements for environmentally friendly materials; they contain no Phosphorus or Nitrogen and are noted for their non-toxic, renewable, and biodegradable properties. Carboxymethyl inulin (CMI), which is isolated from the roots of Inula helenium
Elecampane (''Inula helenium''), pronounced and also called horse-heal or elfdock, is a widespread plant species in the sunflower family Asteraceae. It is native to Eurasia from Spain to Xinjiang province in western China, and naturalized in ...
has been used in oil exploration and its very low toxicity and crystal growth inhibition power has been reported for treating calcite scales. Examples of poorly biodegradable SIs such as the amino-phosphonate and acrylate-based SIs are being phased-out by stringent environmental regulations as demonstrated in the North sea by Norway zero discharge policy.
Another modern alternative to SI use for environmental protection is the development of materials or coatings that intrinsically resist formation of inorganic scale to begin with. A variety of strategies can be used to accomplish this aim, including engineering of wettability properties and engineering of epitaxial properties to prevent mineral growth or to make minerals easier to remove following growth. Recent work has demonstrated that some classes of hydrophobic and superhydrophobic surfaces can cause self-ejection of scale grown during evaporation {{Cite journal, last1=McBride, first1=Samantha, last2=Lake, first2=John, last3=Varanasi, first3=Kripa, date=2023-04-07, title=Self-ejection of salts and other foulants from superhydrophobic surfaces to enable sustainable anti-fouling, journal=The Journal of Chemical Physics, volume=158, issue=13 , pages=134721 , doi=10.1063/5.0142428, pmid=37031132 , doi-access=free
References