O-Cresolphthalein on:
[Wikipedia]
[Google]
[Amazon]
''o''-Cresolphthalein is a
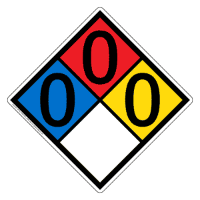
To the left is the NFPA diamond as determined by the Safety Data Sheet, or SDS, by Fisher Scientific. There is minimal risk in handling the chemical.
phthalein dye
Phthalein dyes are a class of dyes mainly used as pH indicators, due to their ability to change colors depending on pH. They are formed by the reaction of phthalic anhydride with various phenols. They are a subclass of triarylmethane dyes.
Common ...
used as a pH indicator in titrations. It is insoluble in water but soluble in ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl ...
. Its solution is colourless below pH 8.2, and purple above 9.8. Its molecular formula is C22H18O4. It is used medically to determine calcium levels in the human body, or to synthesize polyamides or polyimides.
Production
o-Cresolphthalein is not produced industrially, rather, it is commercially available. To be produced, the method generally used to synthesize phthalein dyes is effective. This method is used to synthesize phenolphthalein andthymolphthalein
Thymolphthalein is a phthalein dye used as an acid– base ( pH) indicator. Its transition range is around pH 9.3–10.5. Below this pH, it is colorless; above, it is blue. The molar extinction coefficient for the blue thymolphthalein dianion is ...
. To begin, a 2M equivalent of a phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it req ...
or a substituted phenol should be combined with a 1M equivalent of a phthalic anhydride. However, if the phenol is substituted, the carbon para to the hydroxyl carbon must not be substituted. Anhydrous methanesulfonic acid
Methanesulfonic acid (MsOH) or methanesulphonic acid (in British English) is an organosulfuric, colorless liquid with the chemical formula and structure . It is the simplest of the alkylsulfonic acids (). Salts and esters of methanesulfonic aci ...
should be present as a condensing agent and a solvent that will dehydrate. The combined compounds should be mixed at 90 °C for 5 hours. The temperature should not reach 100 °C at any point. The mixture should then be cooled to room temperature and, once precipitated, added to ice water. The product should then be filtered, washed, and dried. The crude product should then be recrystallized with an appropriate solvent with charcoal treatment-furnished pure phthalein dyes. The color after recrystallization should be between a light brown and a soft yellow.
Uses
The compound has uses ranging from medicine to laboratory syntheses of chemically similar compounds. o-Cresophthalein has been used to derivepolyamide
A polyamide is a polymer with repeating units linked by amide bonds.
Polyamides occur both naturally and artificially. Examples of naturally occurring polyamides are proteins, such as wool and silk. Artificially made polyamides can be made through ...
s and polyimide
Polyimide (sometimes abbreviated PI) is a polymer containing imide groups belonging to the class of high-performance plastics. With their high heat-resistance, polyimides enjoy diverse applications in roles demanding rugged organic materials, e.g ...
s, colorimetrically estimate calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to ...
in serum, and predict amount of time to wait before blood collection after a patient receives gadodiamide
Gadodiamide, sold under the brand name Omniscan, is a gadolinium-based MRI contrast agent (GBCA), used in magnetic resonance imaging (MRI) procedures to assist in the visualization of blood vessels.
Medical uses
Gadodiamide is a contrast med ...
.
Deriving Polyamides and Polyimides
Aromatic polyamides and polyamides are practical compounds due to their temperature resistance, electrical or insulating characteristics, and their mechanical strength. Some of the polyamides and polyimides that can be synthesized by o-Cresophthalein arepolycarbonate
Polycarbonates (PC) are a group of thermoplastic polymers containing carbonate groups in their chemical structures. Polycarbonates used in engineering are strong, tough materials, and some grades are optically transparent. They are easily work ...
, polyacrylate, and epoxy-resin.
The diether-diamine 3,3-bis -(4-amino-phenoxy)-3-methylphenylhthalide, or BNMP, is synthesized by 12 g o-cresophthalein, 11.5 g ''p''-chloronitrobenzene, 5.1 g anhydrous potassium carbonate, and 55 mL of DMF. The compounds should be refluxed together at 160 °C for eight hours. Once it is done and has cooled, it should be mixed with 0.3 L methanol. A precipitate should form and be vacuum filtered to obtain a solid. It should then be washed with water and dried, yielding a yellow product. It should then be recrystallized from glacial acetic acid to yield yellow needles. The product is BNMP. The reaction can go further by combining 15.5 g of BNMP with 0.18 g 10% Pd/C and 50 mL ethanol. They should be stirred at 80 °C. 7 mL of hydrazine monohydrate should be added drop by drop for one hour. The solution should then be mixed for eight hours. It should then be filtered to separated from the Pd/C and concentrated. The concentrated solution should be added to water, and a precipitate should be formed. It should then be vacuum filtered to isolate the solid, yelding 3,3-Bis -(4-aminophenoxy)-3-methylphenylthalide, or BAMP, as a white solid. It should then be purified by water and ethanol.
Colorimetric Estimation of Calcium in Serum
Calcium in a blood sample should be estimated when required medically. Calcium should be precipitated out of 0.1 mL of the blood sample serum as calcium oxalate. After that, the decomposition of the calcium oxalate should occur by heat. Then, the sample should be estimated colorimetrically by o-cresolphthalein complexone. The required liquid complexone is made by dissolving 10 mg o-cresolphthalein complexone in 50 mL alkaline borate, and then 50 mL of 0.05 N HCl are added to make the solution's pH 8.5. This method for calcium determination is efficient and effective, requiring a minimal amount of blood serum sample and a reasonable amount of time.Determination of Impact of Gadodiamide on Calcium Measurements
Gadolinium is given to patients for magnetic resonance imaging, or an MRI.It is used as a contrast agent for the exam to improve clarity of the images formed. However, it can react in the human body and have detrimental effects. Therefore, the agent should be removed. One of these gadolinium based agents is gadodiamide. Calcium in the body should be determined accurately to ensure that the Gadodiamide does not have adverse effects on the patient. There are two o-cresolphthalein methods to determine amount of calcium. The o-cresolpthalein methods are effective because it is a calcium binding dye. The gadolinium ion with a charge of +3 can be removed from gadodiamide using o-cresolphthalein. For these methods, glomerular filtration rate, or GFR, and time since gadodiamide was given should be recorded. Ultimately, these two factors and the impact of gadodiamide on calcium levels calculated by the o-cresolphthalein method helps to reveal an amount of time that patients must wait after receiving gadodiamide to have blood drawn again, or avoid pseudohypocalcemia.Safety
NFPA Diamond

To the left is the NFPA diamond as determined by the Safety Data Sheet, or SDS, by Fisher Scientific. There is minimal risk in handling the chemical.
References
External links
*https://web.archive.org/web/20150924095330/http://www.sciencelab.com/msds.php?msdsId=9923574 *http://www.chemicaldictionary.org/dic/O/o-Cresolphthalein_1298.html *http://www.chemspider.com/Chemical-Structure.62217.html {{DEFAULTSORT:Cresolphthalein2 PH indicators Triarylmethane dyes