Nitrogen trifluoride on:
[Wikipedia]
[Google]
[Amazon]
Nitrogen trifluoride () is an inorganic, colorless, non- flammable,
 is a greenhouse gas, with a global warming potential (GWP) 17,200 times greater than that of when compared over a 100-year period.
Its GWP place it second only to in the group of Kyoto-recognised greenhouse gases, and was included in that grouping with effect from 2013 and the commencement of the second commitment period of the Kyoto Protocol. It has an estimated atmospheric lifetime of 740 years, although other work suggests a slightly shorter lifetime of 550 years (and a corresponding GWP of 16,800).
Although has a high GWP, for a long time its
is a greenhouse gas, with a global warming potential (GWP) 17,200 times greater than that of when compared over a 100-year period.
Its GWP place it second only to in the group of Kyoto-recognised greenhouse gases, and was included in that grouping with effect from 2013 and the commencement of the second commitment period of the Kyoto Protocol. It has an estimated atmospheric lifetime of 740 years, although other work suggests a slightly shorter lifetime of 550 years (and a corresponding GWP of 16,800).
Although has a high GWP, for a long time its  Since 1992, when less than 100 tons were produced, production has grown to an estimated 4000 tons in 2007 and is projected to increase significantly. World production of NF3 is expected to reach 8000 tons a year by 2010. By far the world's largest producer of is the US industrial gas and chemical company Air Products & Chemicals. An estimated 2% of produced is released into the atmosphere. Robson projected that the maximum atmospheric concentration is less than 0.16 parts per trillion (ppt) by volume, which will provide less than 0.001 Wm−2 of IR forcing.
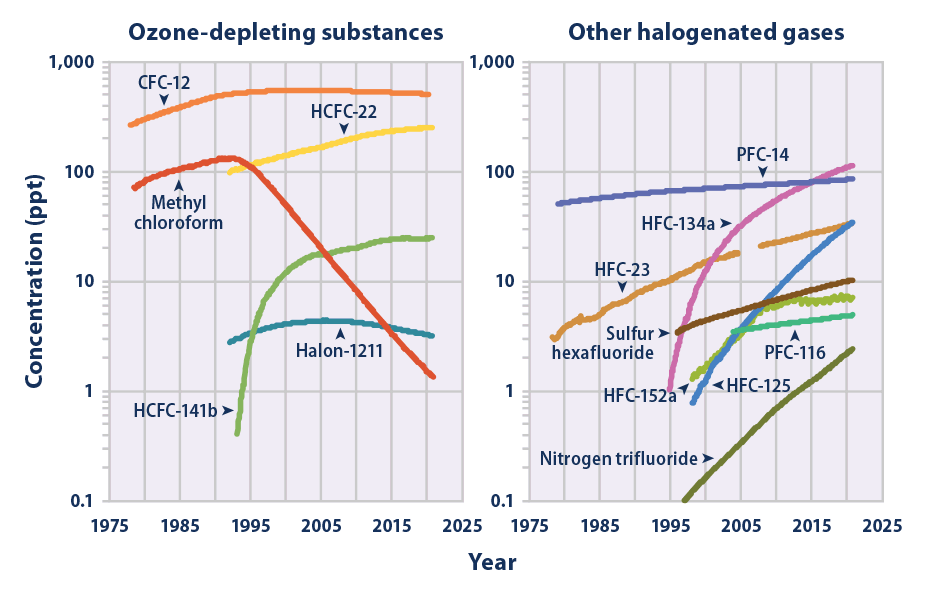
The mean global tropospheric concentration of NF3 has risen from about 0.02 ppt (parts per trillion, dry air mole fraction) in 1980, to 0.86 ppt in 2011, with a rate of increase of 0.095 ppt yr−1, or about 11% per year, and an interhemispheric gradient that is consistent with emissions occurring overwhelmingly in the Northern Hemisphere, as expected. This rise rate in 2011 corresponds to about 1200 metric tons/y NF3 emissions globally, or about 10% of the NF3 global production estimates. This is a significantly higher percentage than has been estimated by industry, and thus strengthens the case for inventorying NF3 production and for regulating its emissions.
One study co-authored by industry representatives suggests that the contribution of the NF3 emissions to the overall greenhouse gas budget of thin-film Si-solar cell manufacturing is clear.
The UNFCCC, within the context of the Kyoto Protocol, decided to include nitrogen trifluoride in the second Kyoto Protocol compliance period, which begins in 2012 and ends in either 2017 or 2020. Following suit, the WBCSD/WRI GHG Protocol is amending all of its standards (corporate, product and Scope 3) to also cover NF3.
Since 1992, when less than 100 tons were produced, production has grown to an estimated 4000 tons in 2007 and is projected to increase significantly. World production of NF3 is expected to reach 8000 tons a year by 2010. By far the world's largest producer of is the US industrial gas and chemical company Air Products & Chemicals. An estimated 2% of produced is released into the atmosphere. Robson projected that the maximum atmospheric concentration is less than 0.16 parts per trillion (ppt) by volume, which will provide less than 0.001 Wm−2 of IR forcing.
The mean global tropospheric concentration of NF3 has risen from about 0.02 ppt (parts per trillion, dry air mole fraction) in 1980, to 0.86 ppt in 2011, with a rate of increase of 0.095 ppt yr−1, or about 11% per year, and an interhemispheric gradient that is consistent with emissions occurring overwhelmingly in the Northern Hemisphere, as expected. This rise rate in 2011 corresponds to about 1200 metric tons/y NF3 emissions globally, or about 10% of the NF3 global production estimates. This is a significantly higher percentage than has been estimated by industry, and thus strengthens the case for inventorying NF3 production and for regulating its emissions.
One study co-authored by industry representatives suggests that the contribution of the NF3 emissions to the overall greenhouse gas budget of thin-film Si-solar cell manufacturing is clear.
The UNFCCC, within the context of the Kyoto Protocol, decided to include nitrogen trifluoride in the second Kyoto Protocol compliance period, which begins in 2012 and ends in either 2017 or 2020. Following suit, the WBCSD/WRI GHG Protocol is amending all of its standards (corporate, product and Scope 3) to also cover NF3.
NF3
Code of Practice (European Industrial Gas Association)]
WebBook page for NF3
{{DEFAULTSORT:Nitrogen Trifluoride Inorganic amines Nitrogen fluorides Greenhouse gases Industrial gases Nitrogen(III) compounds
toxic
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a sub ...
gas with a slightly musty odor. It finds increasing use within the manufacturing of flat-panel displays, photovoltaics, LEDs and other microelectronics
Microelectronics is a subfield of electronics. As the name suggests, microelectronics relates to the study and manufacture (or microfabrication) of very small electronic designs and components. Usually, but not always, this means micrometre- ...
. Nitrogen trifluoride is also an extremely strong and long-lived greenhouse gas. Its atmospheric burden exceeded 2 parts per trillion during 2019 and has doubled every five years since the late 20th century.
Synthesis and reactivity
Nitrogen trifluoride did not exist in significant quantities on Earth prior to its synthesis by humans. It is a rare example of a binary fluoride that can be prepared directly from the elements only at very uncommon conditions, such as an electric discharge. After first attempting the synthesis in 1903, Otto Ruff prepared nitrogen trifluoride by the electrolysis of a molten mixture of ammonium fluoride and hydrogen fluoride. It proved to be far less reactive than the other nitrogen trihalides nitrogen trichloride,nitrogen tribromide
Nitrogen tribromide is a chemical compound with the formula NBr3. It is extremely explosive in its pure form, even at −100 °C, and was not isolated until 1975. It is a deep-red and volatile solid.
Preparation
NBr3 was first prepared by r ...
and nitrogen triiodide, all of which are explosive. Alone among the nitrogen trihalides it has a negative enthalpy of formation. It is prepared in modern times both by direct reaction of ammonia and fluorine and by a variation of Ruff's method.Philip B. Henderson, Andrew J. Woytek "Fluorine Compounds, Inorganic, Nitrogen" in Kirk‑Othmer ''Encyclopedia of Chemical Technology'', 1994, John Wiley & Sons, NY. Article Online Posting Date: December 4, 2000 It is supplied in pressurized cylinders.
is slightly soluble in water without undergoing chemical reaction. It is nonbasic with a low dipole moment of 0.2340 D. By contrast, ammonia is basic and highly polar (1.47 D). This difference arises from the fluorine atoms acting as electron withdrawing groups, attracting essentially all of the lone pair electrons on the nitrogen atom. NF3 is a potent yet sluggish oxidizer.
It oxidizes hydrogen chloride to chlorine:
:2 NF3 + 6 HCl → 6 HF + N2 + 3 Cl2
It is compatible with steel and Monel, as well as several plastics.
It converts to tetrafluorohydrazine upon contact with metals, but only at high temperatures:
:2 NF3 + Cu → N2F4 + CuF2
NF3 reacts with fluorine and antimony pentafluoride to give the tetrafluoroammonium salt:
: NF3 + F2 + SbF5 → NFSbF
Mixtures of NF3 and B2H6 are explosive even at cryogenic temperatures, reacting to produce nitrogen gas, boron trifluoride, and hydrofluoric acid.
Applications
Etching
Nitrogen trifluoride is primarily used to removesilicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic ...
and silicon-compounds during the manufacturing of semiconductor devices such as LCD display
A liquid-crystal display (LCD) is a flat-panel display or other electronically modulated optical device that uses the light-modulating properties of liquid crystals combined with polarizers. Liquid crystals do not emit light directly but in ...
s, some thin-film solar cells, and other microelectronics. In these applications is initially broken down within a plasma. The resulting fluorine radicals
Radical may refer to:
Politics and ideology Politics
*Radical politics, the political intent of fundamental societal change
*Radicalism (historical), the Radical Movement that began in late 18th century Britain and spread to continental Europe and ...
are the active agents that attack polysilicon
Polycrystalline silicon, or multicrystalline silicon, also called polysilicon, poly-Si, or mc-Si, is a high purity, polycrystalline form of silicon, used as a raw material by the solar photovoltaic and electronics industry.
Polysilicon is produce ...
, silicon nitride and silicon oxide Silicon oxide may refer to either of the following:
* Silicon dioxide or quartz, SiO2, very well characterized
*Silicon monoxide
Silicon monoxide is the chemical compound with the formula SiO where silicon is present in the oxidation state +2. In ...
. They can be used as well to remove tungsten silicide
Tungsten silicide (WSi2) is an inorganic compound, a silicide of tungsten. It is an electrically conductive ceramic material.
Chemistry
Tungsten silicide can react violently with substances such as strong acids, fluorine, oxidizers, and interh ...
, tungsten
Tungsten, or wolfram, is a chemical element with the symbol W and atomic number 74. Tungsten is a rare metal found naturally on Earth almost exclusively as compounds with other elements. It was identified as a new element in 1781 and first isol ...
, and certain other metals. In addition to serving as an etchant
Etching is a printmaking technique in art.
Etching may also refer to:
* Etching (microfabrication), a process in producing microelectronics
* Glass etching, a glass decoration technique
* Chemical milling, or industrial etching
* Photochemical ma ...
in device fabrication, is also widely used to clean PECVD chambers.
dissociates more readily within a low-pressure discharge
Low-pressure discharges exist within Gas-discharge lamps. The electric discharges in gases are made under gas pressures from a few millitorr to a little less than atmospheric.
Description
They are most often used in industry to generate plasma ...
in comparison to perfluorinated compounds (PFCs) and sulfur hexafluoride (). The greater abundance of negatively-charged free radicals thus generated can yield higher silicon removal rates, and provide other process benefits such as less residual contamination and a lower net charge stress on the device being fabricated. As a somewhat more thoroughly consumed etching and cleaning agent, NF3 has also been promoted as an environmentally preferable substitute for or PFCs such as hexafluoroethane.
The utilization efficiency of the chemicals applied in plasma processes varies widely between equipment and applications. A sizeable fraction of the reactants are wasted into the exhaust stream and can ultimately be emitted into Earth's atmosphere. Modern abatement systems can substantially decrease atmospheric emissions. has not been subject to significant use restrictions. The annual reporting of production, consumption, and waste emissions by large manufacturers has been required in many industrialized countries as a response to the observed atmospheric growth and the international Kyoto Protocol.
Highly toxic fluorine gas (F2, diatomic fluorine) is a climate neutral
Carbon neutrality is a state of net-zero carbon dioxide emissions. This can be achieved by balancing emissions of carbon dioxide with its removal (often through carbon offsetting) or by eliminating emissions from society (the transition to the " ...
replacement for nitrogen trifluoride in some manufacturing applications. It requires more stringent handling and safety precautions, especially to protect manufacturing personnel.
Nitrogen trifluoride is also used in hydrogen fluoride and deuterium fluoride lasers, which are types of chemical lasers. There it is also preferred to fluorine gas due to its more convenient handling properties
Greenhouse gas
 is a greenhouse gas, with a global warming potential (GWP) 17,200 times greater than that of when compared over a 100-year period.
Its GWP place it second only to in the group of Kyoto-recognised greenhouse gases, and was included in that grouping with effect from 2013 and the commencement of the second commitment period of the Kyoto Protocol. It has an estimated atmospheric lifetime of 740 years, although other work suggests a slightly shorter lifetime of 550 years (and a corresponding GWP of 16,800).
Although has a high GWP, for a long time its
is a greenhouse gas, with a global warming potential (GWP) 17,200 times greater than that of when compared over a 100-year period.
Its GWP place it second only to in the group of Kyoto-recognised greenhouse gases, and was included in that grouping with effect from 2013 and the commencement of the second commitment period of the Kyoto Protocol. It has an estimated atmospheric lifetime of 740 years, although other work suggests a slightly shorter lifetime of 550 years (and a corresponding GWP of 16,800).
Although has a high GWP, for a long time its radiative forcing
Radiative forcing (or climate forcing) is the change in energy flux in the atmosphere caused by natural or anthropogenic factors of climate change as measured by watts / metre2. It is a scientific concept used to quantify and compare the extern ...
in the Earth's atmosphere
The atmosphere of Earth is the layer of gases, known collectively as air, retained by Earth's gravity that surrounds the planet and forms its planetary atmosphere. The atmosphere of Earth protects life on Earth by creating pressure allowing fo ...
has been assumed to be small, spuriously presuming that only small quantities are released into the atmosphere. Industrial applications of routinely break it down, while in the past previously used regulated compounds such as and PFCs were often released. Research has questioned the previous assumptions. High-volume applications such as DRAM computer memory production, the manufacturing of flat panel displays and the large-scale production of thin-film solar cells use .
 Since 1992, when less than 100 tons were produced, production has grown to an estimated 4000 tons in 2007 and is projected to increase significantly. World production of NF3 is expected to reach 8000 tons a year by 2010. By far the world's largest producer of is the US industrial gas and chemical company Air Products & Chemicals. An estimated 2% of produced is released into the atmosphere. Robson projected that the maximum atmospheric concentration is less than 0.16 parts per trillion (ppt) by volume, which will provide less than 0.001 Wm−2 of IR forcing.
The mean global tropospheric concentration of NF3 has risen from about 0.02 ppt (parts per trillion, dry air mole fraction) in 1980, to 0.86 ppt in 2011, with a rate of increase of 0.095 ppt yr−1, or about 11% per year, and an interhemispheric gradient that is consistent with emissions occurring overwhelmingly in the Northern Hemisphere, as expected. This rise rate in 2011 corresponds to about 1200 metric tons/y NF3 emissions globally, or about 10% of the NF3 global production estimates. This is a significantly higher percentage than has been estimated by industry, and thus strengthens the case for inventorying NF3 production and for regulating its emissions.
One study co-authored by industry representatives suggests that the contribution of the NF3 emissions to the overall greenhouse gas budget of thin-film Si-solar cell manufacturing is clear.
The UNFCCC, within the context of the Kyoto Protocol, decided to include nitrogen trifluoride in the second Kyoto Protocol compliance period, which begins in 2012 and ends in either 2017 or 2020. Following suit, the WBCSD/WRI GHG Protocol is amending all of its standards (corporate, product and Scope 3) to also cover NF3.
Since 1992, when less than 100 tons were produced, production has grown to an estimated 4000 tons in 2007 and is projected to increase significantly. World production of NF3 is expected to reach 8000 tons a year by 2010. By far the world's largest producer of is the US industrial gas and chemical company Air Products & Chemicals. An estimated 2% of produced is released into the atmosphere. Robson projected that the maximum atmospheric concentration is less than 0.16 parts per trillion (ppt) by volume, which will provide less than 0.001 Wm−2 of IR forcing.
The mean global tropospheric concentration of NF3 has risen from about 0.02 ppt (parts per trillion, dry air mole fraction) in 1980, to 0.86 ppt in 2011, with a rate of increase of 0.095 ppt yr−1, or about 11% per year, and an interhemispheric gradient that is consistent with emissions occurring overwhelmingly in the Northern Hemisphere, as expected. This rise rate in 2011 corresponds to about 1200 metric tons/y NF3 emissions globally, or about 10% of the NF3 global production estimates. This is a significantly higher percentage than has been estimated by industry, and thus strengthens the case for inventorying NF3 production and for regulating its emissions.
One study co-authored by industry representatives suggests that the contribution of the NF3 emissions to the overall greenhouse gas budget of thin-film Si-solar cell manufacturing is clear.
The UNFCCC, within the context of the Kyoto Protocol, decided to include nitrogen trifluoride in the second Kyoto Protocol compliance period, which begins in 2012 and ends in either 2017 or 2020. Following suit, the WBCSD/WRI GHG Protocol is amending all of its standards (corporate, product and Scope 3) to also cover NF3.
Safety
Skin contact with is not hazardous, and it is a relatively minor irritant to mucous membranes and eyes. It is a pulmonary irritant with atoxicity
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a subs ...
considerably lower than nitrogen oxides, and overexposure via inhalation causes the conversion of hemoglobin
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythroc ...
in blood to methemoglobin, which can lead to the condition methemoglobinemia. The National Institute for Occupational Safety and Health (NIOSH) specifies that the concentration that is immediately dangerous to life or health (IDLH value) is 1,000 ppm.
See also
* IPCC list of greenhouse gases * Nitrogen pentafluoride * TetrafluorohydrazineNotes
References
External links
*NF3
Code of Practice (European Industrial Gas Association)]
WebBook page for NF3
{{DEFAULTSORT:Nitrogen Trifluoride Inorganic amines Nitrogen fluorides Greenhouse gases Industrial gases Nitrogen(III) compounds