Narcan on:
[Wikipedia]
[Google]
[Amazon]
Naloxone, sold under the brand names Narcan (4 mg) and Kloxxado (8 mg) among others, is a medication used to reverse or reduce the effects of
 Naloxone is useful in treating both acute
Naloxone is useful in treating both acute

. Retrieved 29 February 2016. In Europe, take home naloxone pilots were launched in the Channel Islands and in Berlin in the late 1990s. In 2008 the Welsh Assembly government announced its intention to establish demonstration sites for take-home naloxone, and in 2010 Scotland instituted a national naloxone program. Inspired by North American and European efforts, non-governmental organizations running programs to train drug users as overdose responders and supply them with naloxone are now operational in Russia, Ukraine, Georgia, Kazakhstan, Tajikistan, Afghanistan, China, Vietnam, and Thailand. Noting the high risk of overdose among people with HIV who inject drugs, international HIV donors including the President's Emergency Plan for AIDS Relief, the The Global Fund to Fight AIDS, Tuberculosis and Malaria, Global Fund to Fight AIDS, Tuberculosis and Malaria, and the Open Society Foundations, have supported the purchase and distribution of naloxone to those at risk in low- and middle income countries. In 2017, Next Harm Reduction in New York State began distributing naloxone and other harm reduction supplies by mail to those in the US unable to get them locally. In 2018, a maker of naloxone announced it would provide a free kit including two doses of the nasal spray, as well as educational materials, to each of the 16,568 public libraries and 2,700 YMCAs in the U.S.
opioids
Opioids are substances that act on opioid receptors to produce morphine-like effects. Medically they are primarily used for pain relief, including anesthesia. Other medical uses include suppression of diarrhea, replacement therapy for opioid use ...
. It is commonly used to counter decreased breathing in opioid overdose
An opioid overdose is toxicity due to excessive consumption of opioids, such as morphine, codeine, heroin, fentanyl, tramadol, and methadone. This preventable pathology can be fatal if it leads to respiratory depression, a lethal condition that ca ...
. Effects begin within two minutes when given intravenously
Intravenous therapy (abbreviated as IV therapy) is a medical technique that administers fluids, medications and nutrients directly into a person's vein. The intravenous route of administration is commonly used for rehydration or to provide nutrie ...
, and within five minutes when injected into a muscle. The medicine can also be administered by spraying it into a person's nose. Naloxone commonly blocks the effects of opioids for 30 to 90 minutes. Multiple doses may be required, as the duration of action of some opioids is greater than that of naloxone.
Administration to opioid-dependent individuals may cause symptoms of opioid withdrawal
Opioid withdrawal is a set of symptoms (a syndrome) arising from the sudden withdrawal or reduction of opioids where previous usage has been heavy and prolonged. Signs and symptoms of withdrawal can include drug craving, anxiety, restless legs, ...
, including restlessness, agitation, nausea, vomiting, a fast heart rate
Tachycardia, also called tachyarrhythmia, is a heart rate that exceeds the normal resting rate. In general, a resting heart rate over 100 beats per minute is accepted as tachycardia in adults. Heart rates above the resting rate may be normal (su ...
, and sweating. To prevent this, small doses every few minutes can be given until the desired effect is reached. In those with previous heart disease or taking medications that negatively affect the heart, further heart problems have occurred. It appears to be safe in pregnancy, after having been given to a limited number of women. Naloxone is a non-selective
In biochemistry and pharmacology, a ligand is a Chemical substance, substance that forms a Complex (chemistry), complex with a biomolecule to serve a biological purpose. The etymology stems from ''ligare'', which means 'to bind'. In protein-lig ...
and competitive
Competition is a rivalry where two or more parties strive for a common goal which cannot be shared: where one's gain is the other's loss (an example of which is a zero-sum game). Competition can arise between entities such as organisms, indivi ...
opioid receptor antagonist
An opioid antagonist, or opioid receptor antagonist, is a receptor antagonist that acts on one or more of the opioid receptors.
Naloxone and naltrexone are commonly used opioid antagonist drugs which are competitive antagonists that bind to the o ...
. It works by reversing the depression of the central nervous system and respiratory system caused by opioids. Naloxone generally has no effect on those not using opioids, has no abuse potential, and is recommended by the World Health Organization for distribution to anyone likely to encounter a fatal opioid overdose, including emergency personnel and friends and family members of those using opioids.
Naloxone was patented in 1961 and approved for opioid overdose in the United States in 1971. It is on the World Health Organization's List of Essential Medicines
The WHO Model List of Essential Medicines (aka Essential Medicines List or EML), published by the World Health Organization (WHO), contains the medications considered to be most effective and safe to meet the most important needs in a health ...
. Naloxone is available as a generic medication
A generic drug is a pharmaceutical drug that contains the same chemical substance as a drug that was originally protected by chemical patents. Generic drugs are allowed for sale after the patents on the original drugs expire. Because the active ch ...
. In April 2021, the U.S. Food and Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is respon ...
(FDA) approved a higher dose naloxone hydrochloride nasal spray product (''Kloxxado'') intended to treat opioid overdose from fentanyl
Fentanyl, also spelled fentanil, is a very potent synthetic opioid used as a pain medication. Together with other drugs, fentanyl is used for anesthesia. It is also used illicitly as a recreational drug, sometimes mixed with heroin, cocaine ...
and its analogues, which are many times stronger than heroin.
Medical uses
Opioid overdose
 Naloxone is useful in treating both acute
Naloxone is useful in treating both acute opioid overdose
An opioid overdose is toxicity due to excessive consumption of opioids, such as morphine, codeine, heroin, fentanyl, tramadol, and methadone. This preventable pathology can be fatal if it leads to respiratory depression, a lethal condition that ca ...
and respiratory or mental depression due to opioids. Whether it is useful in those in cardiac arrest
Cardiac arrest is when the heart suddenly and unexpectedly stops beating. It is a medical emergency that, without immediate medical intervention, will result in sudden cardiac death within minutes. Cardiopulmonary resuscitation (CPR) and possib ...
due to an opioid overdose is unclear.
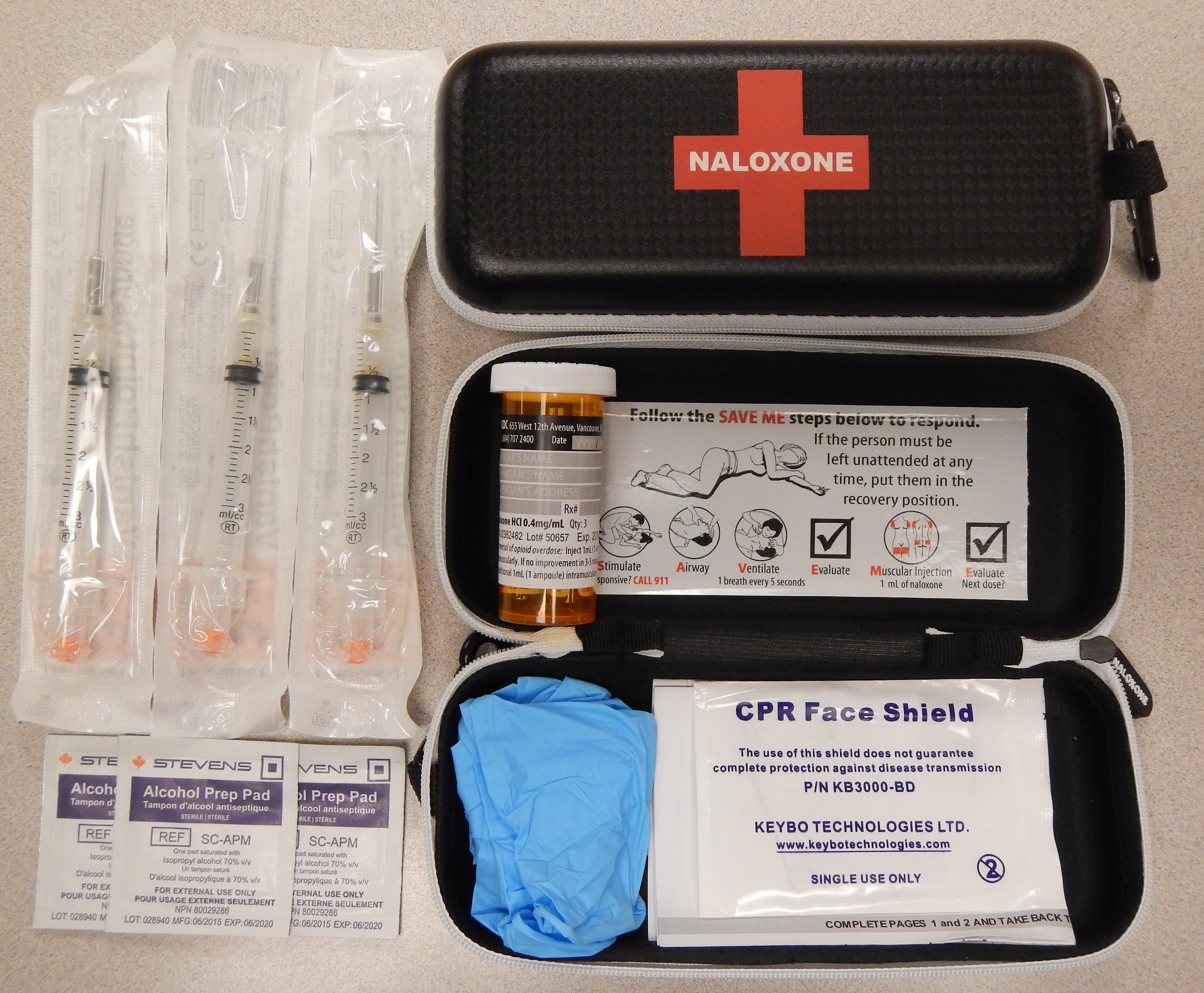
It is included as a part of emergency overdose response kits distributed to heroin
Heroin, also known as diacetylmorphine and diamorphine among other names, is a potent opioid mainly used as a recreational drug for its euphoric effects. Medical grade diamorphine is used as a pure hydrochloride salt. Various white and brow ...
and other opioid drug users, and to emergency responders. This has been shown to reduce rates of deaths due to overdose. A prescription for naloxone is recommended if a person is on a high dose of opioid (>100 mg of morphine equivalence/day), is prescribed any dose of opioid accompanied by a benzodiazepine
Benzodiazepines (BZD, BDZ, BZs), sometimes called "benzos", are a class of depressant drugs whose core chemical structure is the fusion of a benzene ring and a diazepine ring. They are prescribed to treat conditions such as anxiety disorders, ...
, or is suspected or known to use opioids nonmedically. Prescribing naloxone should be accompanied by standard education that includes preventing, identifying, and responding to an overdose; rescue breathing; and calling emergency services.
Distribution of naloxone to individuals likely to encounter people who overdose is part of the harm reduction
Harm reduction, or harm minimization, refers to a range of public health policies designed to lessen the negative social and/or physical consequences associated with various human behaviors, both legal and illegal. Harm reduction is used to de ...
initiatives that have spread throughout the US and the world. This approach to dealing with substance use disorder
Substance use disorder (SUD) is the persistent use of drugs (including alcohol) despite substantial harm and adverse consequences as a result of their use. Substance use disorders are characterized by an array of mental/emotional, physical, and ...
is to treat it as a medical problem and focusing efforts on reducing the harm produced directly (e.g., overdose) and indirectly (exposure to infectious disease).
Clonidine overdose
Naloxone can also be used as an antidote in overdose ofclonidine
Clonidine, sold under the brand name Catapres among others, is an α2-adrenergic agonist medication used to treat high blood pressure, ADHD, drug withdrawal ( alcohol, opioids, or nicotine), menopausal flushing, diarrhea, spasticity, and c ...
, a medication that lowers blood pressure. Clonidine overdoses are of special relevance for children, in whom even small doses can cause significant harm. However, there is controversy regarding naloxone's efficacy in treating the symptoms of clonidine overdose, namely bradycardia, slow heart rate, hypotension, low blood pressure, and altered mental status, confusion/somnolence. Case reports that used doses of 0.1 mg/kg (maximum of 2 mg/dose) repeated every 1–2 minutes (10 mg total dose) have shown inconsistent benefit. As the doses used throughout the literature vary, it is difficult to form a conclusion regarding the benefit of naloxone in this setting. The mechanism for naloxone's proposed benefit in clonidine overdose is unclear, but it has been suggested that endogenous opioid receptors mediate the sympathetic nervous system in the brain and elsewhere in the body. Some poison control centers recommend naloxone in the setting of clonidine overdose, including intravenous bolus doses of up to 10 mg naloxone.
Preventing recreational opioid use
Naloxone is poorly absorbed when taken by mouth, so it is commonly combined with a number of oral opioid preparations, including buprenorphine and pentazocine, so that when taken by mouth, only the opioid has an effect. However, if the opioid and naloxone combination is injected, the naloxone blocks the effect of the opioid. This combination is used in an effort to prevent non-medical use.Other uses
Naloxone can be used to treat opioid induced itchiness and constipation. A 2003 meta-analysis of existing research showed naloxone to improve blood flow in patients with circulatory shock, shock, including septic shock, septic, cardiogenic, hemorrhagic, or spinal shock, but could not determine if this reduced patient deaths. Naloxone has been used experimentally in the treatment of congenital insensitivity to pain with anhidrosis.Special populations
Pregnancy and breastfeeding
Naloxone is pregnancy category B or C in the United States. Studies in rodents given a daily maximum dose of 10 mg naloxone showed no harmful effects to the fetus, although human studies are lacking and the drug does cross the placenta, which may lead to the precipitation of withdrawal in the fetus. In this setting, further research is needed before safety can be assured, so naloxone should be used during pregnancy only if it is a medical necessity. Whether naloxone is excreted in breast milk is unknown, however, it is not Bioavailability, orally bioavailable and therefore is unlikely to affect a breastfeeding infant.Children
Naloxone can be used on infants who were exposed to intrauterine opiates administered to mothers during delivery. However, there is insufficient evidence for the use of naloxone to lower cardiorespiratory and neurological depression in these infants. Infants exposed to high concentrations of opiates during pregnancy may have CNS damage in the setting of perinatal asphyxia. Naloxone has been studied to improve outcomes in this population, however the evidence is currently weak. Intravenous, intramuscular, or subcutaneous administration of naloxone can be given to children and neonates to reverse opiate effects. The American Academy of Pediatrics recommends only intravenous administration as the other two forms can cause unpredictable absorption. After a dose is given, the child should be monitored for at least 24 hours. For children with low blood pressure due to septic shock, naloxone safety and effectiveness are not established.Geriatric use
For patients 65 years and older, it is unclear if there is a difference in response. However, older people often have decreased liver and kidney function that may lead to an increased level of naloxone in their body.Side effects
Naloxone has little to no effect if opioids are not present. In people with opioids in their system, it may cause increased sweating, nausea, restlessness, trembling, vomiting, flushing, and headache, and has in rare cases been associated with heart rhythm changes, seizures, and pulmonary edema. Besides the side effects listed above, naloxone also has other adverse events, such as other cardiovascular effects (hypertension, hypotension, tachycardia, ventricular fibrillation, ventricular tachycardia) and central nervous system effects, such as agitation, body pain, brain disease, and coma. In addition to these adverse effects, naloxone is also contraindicated in people with hypersensitivity to naloxone or any of its formulation components. Naloxone has been shown to block the action of pain-lowering endorphins the body produces naturally. These endorphins likely operate on the same opioid receptors that naloxone blocks. It is capable of blocking a placebo pain-lowering response, if the placebo is administered together with a hidden or blind injection of naloxone. Other studies have found that placebo alone can activate the body's μ-opioid endorphin system, delivering pain relief by the same receptor mechanism as morphine. Naloxone should be used with caution in people with cardiovascular disease as well as those that are currently taking medications that could have adverse effects on the cardiovascular system such as causing Hypotension, low blood pressure, pulmonary edema, fluid accumulation in the lungs (pulmonary edema), and arrhythmia, abnormal heart rhythms. There have been reports of abrupt reversals with opioid antagonists leading to pulmonary edema and ventricular fibrillation.Hypersensitivities
Naloxone preparations may contain methylparaben and propylparaben and are inappropriate for use by people with a paraben hypersensitivity. If a person is sensitive to nalmefene or naltrexone, naloxone should be used with caution as these three medications are structurally similar. Cross-sensitivity among these drugs is unknown. Preservative-free preparations are available for those with paraben hypersensitivities.Pharmacology
Pharmacodynamics
Naloxone is a lipophilic compound that acts as anon-selective
In biochemistry and pharmacology, a ligand is a Chemical substance, substance that forms a Complex (chemistry), complex with a biomolecule to serve a biological purpose. The etymology stems from ''ligare'', which means 'to bind'. In protein-lig ...
and competitive
Competition is a rivalry where two or more parties strive for a common goal which cannot be shared: where one's gain is the other's loss (an example of which is a zero-sum game). Competition can arise between entities such as organisms, indivi ...
opioid receptor antagonist
An opioid antagonist, or opioid receptor antagonist, is a receptor antagonist that acts on one or more of the opioid receptors.
Naloxone and naltrexone are commonly used opioid antagonist drugs which are competitive antagonists that bind to the o ...
. The eudysmic ratio, pharmacologically active isomer of naloxone is (−)-naloxone. Naloxone's binding affinity is highest for the μ-opioid receptor (MOR), then the δ-opioid receptor (DOR), and lowest for the κ-opioid receptor (KOR); naloxone has negligible affinity for the nociceptin receptor.
If naloxone is administered in the absence of concomitant opioid use, no functional pharmacological activity occurs, except the inability of the body to combat pain naturally. In contrast to direct opiate agonists, which elicit opiate withdrawal symptoms when discontinued in opiate-tolerant people, no evidence indicates the development of tolerance or dependence on naloxone. The mechanism of action is not completely understood, but studies suggest it functions to produce withdrawal symptoms by competing for opioid receptors within the brain (a competitive antagonist, not a direct agonist), thereby preventing the action of both endogenous and xenobiotic opioids on these receptors without directly producing any effects itself.
A single administration of naloxone at a relatively high dose of 2 mg by intravenous injection has been found to produce brain MOR blockade of 80% at 5 minutes, 47% at 2 hours, 44% at 4 hours, and 8% at 8 hours. A low dose (2 μg/kg) produced brain MOR blockade of 42% at 5 minutes, 6% at 2 hours, 33% at 4 hours, and 10% at 8 hours. Intranasal administration of naloxone via nasal spray has likewise been found to rapidly occupy brain MORs, with peak occupancy occurring at 20 minutes, peak occupancies of 67% at a dose of 2 mg and 85% with 4 mg, and an estimated half-life of occupancy disappearance of approximately 100 minutes (1.67 hours).
Pharmacokinetics
When administered wikt:parenteral, parenterally (non-orally or non-rectally, e.g., intravenously or by injection), as is most common, naloxone has a rapid distribution throughout the body. The mean serum half-life has been shown to range from 30 to 81 minutes, shorter than the average half-life of some opiates, necessitating repeat dosing if opioid receptors must be stopped from triggering for an extended period. Naloxone is primarily metabolized by the liver. Its major metabolite is naloxone-3-glucuronide, which is excreted in the urine. For people with liver diseases such as alcoholic liver disease or hepatitis, naloxone usage has not been shown to increase serum liver enzyme levels. Naloxone has low systemic bioavailability when Oral administration, taken by mouth due to hepatic first-pass metabolism, but it does block opioid receptors that are located in the intestine.Chemistry
Naloxone, also known as N-allylnoroxymorphone or as 17-allyl-4,5α-epoxy-3,14-dihydroxymorphinan-6-one, is a synthetic compound, synthetic morphinan chemical derivative, derivative and was derived from oxymorphone (14-hydroxydihydromorphinone), an opioid analgesic. Oxymorphone, in turn, was derived from morphine, an opioid analgesic and natural product, naturally occurring constituent of the opium poppy. Naloxone is a racemic mixture of two enantiomers, (–)-naloxone (levonaloxone) and (+)-naloxone (dextronaloxone), only the former of which is active at opioid receptors. The drug is highly lipophilic, allowing it to rapidly penetrate the brain and to achieve a far greater brain to serum ratio than that of morphine. Opioid antagonists related to naloxone include cyprodime, nalmefene, nalodeine, naloxol, and naltrexone. The chemical half-life of naloxone is such that injection and nasal forms have been marketed with 24-month and 18-month shelf-lives, respectively. A 2018 study noted that the nasal and injection forms presented as chemically stable to 36- and 28-months, respectively, which prompted an as yet incomplete five-year stability study to be initiated. This suggests that expired caches of material in community and healthcare settings may still be efficacious substantially beyond their labeled expiration dates.History
Naloxone was patented in 1961 by Mozes J. Lewenstein, Jack Fishman, and the company Daiichi Sankyo, Sankyo. It was approved for opioid use disorder treatment in the United States in 1971, with opioid overdose prevention kits being distributed by many states to medically untrained people beginning in 1996. From the period of 1996 to 2014, the CDC estimates over 26,000 cases of opioid overdose have been reversed using the kits. Naloxone (Nyxoid) was approved for use in the European Union in September 2017.Society and culture
Names
''Naloxone'' is the generic term, generic name of the medication and its , , , , and , while ''naloxone hydrochloride'' is its and . The patent has expired and it is available as a generic drug, generic medication. Several of the newer formulations use patented dispensers (spray mechanisms or autoinjectors), and patent disputes over generic forms of the nasal spray were litigated between 2016 and 2020, when a judge ruled in favor of Teva, the generic manufacturer. Teva announced entry of the first generic nasal spray formulation in December 2021. Brand names of naloxone include Narcan, Kloxxado, Nalone, Evzio, Prenoxad Injection, Narcanti, Narcotan, and Zimhi, among others.Identification
The CAS number of naloxone is 465-65-6; the anhydrous hydrochloride salt (chemistry), salt has CAS 357-08-4 and the hydrochloride salt with 2 molecules of water, hydrochloride dihydrate, has CAS 51481-60-8Routes of administration
Intravenous
In hospital settings, naloxone is commonly injected intravenously, with an onset of 1–2 minutes and a duration of up to 45 minutes. While the onset is achieved fastest through IV than through other routes of administration, it may be difficult to obtain venous access in patients who use IV drugs chronically. This may be an issue under emergency conditions.Intramuscular or subcutaneous
Naloxone can also be administered via intramuscular or subcutaneous injection. The onset of naloxone provided through this route is 2 to 5 minutes with a duration of around 30-120min.Naloxone for Treatment of Opioid Overdose Oct. 2016 Naloxone administered intramuscularly are provided through pre-filled syringes, vials, and auto-injector. Evzio is the only auto-injector on the market and can be used both intramuscularly and subcutaneously. It is pocket-sized and can be used in non-medical settings such as in the home. It is designed for use by laypersons, including family members and caregivers of opioid users at-risk for an opioid emergency, such as an overdose. According to the FDA's National Drug Code Directory, a generic version of the auto-injector began to be marketed at the end of 2019.
Intranasal
Administration of naloxone intranasally is recommended for people who are unconscious or unresponsive. While the onset of action is slightly delayed in this method of administration, the ease of use and portability are what make naloxone nasal sprays useful. Narcan Nasal Spray was approved in 2015 and was the first FDA-approved nasal spray for emergency treatment or suspected overdose. Narcan Nasal Spray is prepackaged, requires no assembly, and delivers a consistent 4 mg dose of naloxone. It was developed in a partnership between LightLake Therapeutics and the National Institute on Drug Abuse. The approval process was fast-tracked. A generic version of the nasal spray was approved in the United States in 2019, though did not come to market until 2021. In 2021, the FDA approved Kloxxado, a 8 mg dose of intranasal naloxone developed by Hikma Pharmaceuticals. Citing the frequent need for multiple 4 mg doses of Narcan to successfully reverse overdose, packs of Kloxxado Nasal Spray contain two pre-packaged nasal spray devices, each containing 8 mg of naloxone. However, a wedge device (nasal atomizer) can also be attached to a syringe that may also be used to create a mist to deliver the drug to the nasal mucosa. This is useful near facilities where many overdoses occur that already stock injectors.Storage
Naloxone should be stored at room temperature and protected from light. For the auto-injector, naloxone should be stored in the outer case provided. If the product is cloudy, discolored, or contains particulate matter, use is not recommended.Legal status and availability to law enforcement and emergency personnel
In the United States, naloxone is ostensibly available without a prescription in every state with the exception of Hawaii. In reality, not all pharmacies stock or dispense naloxone. Depending on the pharmacy, a pharmacist may have to write a prescription or not be able to give naloxone to comply with accounting rules, as naloxone is still considered a prescription-only medication under FDA rules. As of mid-2019, officials in 29 states had issued standing orders to enable licensed pharmacists to provide naloxone to patients without the individual first visiting a prescriber. Prescribers working with harm reduction or low threshold treatment programs have also issued standing orders to enable these organizations to distribute naloxone to their clients. A standing order, also referred to as a "non-patient specific prescription" is written by a physician, nurse or other prescriber to authorize medicine distribution outside the doctor-patient relationship. In the case of naloxone, these orders are meant to facilitate naloxone distribution to people using opioids, and their family members and friends. Over 200 naloxone distribution programs utilize licensed prescribers to distribute the drug through such orders, or through the authority of pharmacists (as with California's legal proision, AB1535). Laws and policies in many US jurisdictions have been changed in recent years to allow wider distribution of naloxone. In addition to laws or regulations permitting distribution of medicine to at risk individuals and families, some 36 states have passed laws that provide naloxone prescribers with immunity against both civil and criminal liabilities. While paramedics in the US have carried naloxone for decades, law enforcement officers in many states throughout the country carry naloxone to reverse the effects of heroin overdoses when reaching the location before paramedics. As of July 12, 2015, law enforcement departments in 28 US states are allowed to or required to carry naloxone to quickly respond to opioid overdoses. Programs training fire personnel in opioid overdose response using naloxone have also shown promise in the US, and efforts to integrate opioid fatality prevention into emergency response have grown due to the US overdose crisis. Following the use of the nasal spray device by police officers on Staten Island in New York, an additional 20,000 police officers will begin carrying naloxone in mid-2014. The state's Office of the Attorney General will provide US$1.2 million to supply nearly 20,000 kits. Police Commissioner William Bratton said: "Naloxone gives individuals a second chance to get help". Emergency Medical Service Providers (EMS) routinely administer naloxone, except where basic Emergency Medical Technicians are prohibited by policy or by state law. In efforts to encourage citizens to seek help for possible opioid overdoses, many states have adopted Good Samaritan laws that provide immunity against certain criminal liabilities for anybody who, in good faith, seeks emergency medical care for either themselves or someone around them who may be experiencing an opioid overdose. States including Vermont and Virginia have developed programs that mandate the prescription of naloxone when a prescription has exceeded a certain level of morphine milliequivalents per day as preventative measures against overdose. Healthcare institution-based naloxone prescription programs have also helped reduce rates of opioid overdose in North Carolina, and have been replicated in the US military. In Canada, naloxone single-use syringe kits are distributed and available at various clinics and emergency rooms. Alberta Health Services is increasing the distribution points for naloxone kits at all emergency rooms, and various pharmacies and clinics province-wide. All Edmonton Police Service and Calgary Police Service patrol cars carry an emergency single-use naloxone syringe kit. Some Royal Canadian Mounted Police patrol vehicles also carry the drug, occasionally in excess to help distribute naloxone among users and concerned family/friends. Nurses, paramedics, medical technicians, and emergency medical responders can also prescribe and distribute the drug. As of February 2016, pharmacies across Alberta and some other Canadian jurisdictions are allowed to distribute single-use take-home naloxone kits or prescribe the drug to people using opioids. Following Alberta Health Services, Health Canada reviewed the prescription-only status of naloxone, resulting in plans to remove it in 2016, making naloxone more accessible. Due to the rising number of drug deaths across the country, Health Canada proposed a change to make naloxone more widely available to Canadians in support of efforts to address the growing number of opioid overdoses. In March 2016, Health Canada did change the prescription status of naloxone, as "pharmacies are now able to proactively give out naloxone to those who might experience or witness an opioid overdose."Community access
Schools, government agencies, and nonprofit organizations hold training programs to educate laypeople on the proper use of naloxone and to send them home with medicine. It is estimated that programs like these have helped to reverse more than 26,000 overdoses in the US. Harm reduction organizations providing needle and syringes to those injecting drugs have been particularly involved in naloxone distribution, purchasing medicine at discounted rates through a Buyer's Club and distributing more than 3 million vials to those at high risk or likely to be with those at high risk between 2017 and 2020 alone. In a survey of US laypersons in December 2021, most people believed the scientifically-supported idea that trained bystanders can reverse overdoses with naloxone. A survey of US naloxone prescription programs in 2010 revealed that 21 out of 48 programs reported challenges in obtaining naloxone in the months leading up to the survey, due mainly to either cost increases that outstripped allocated funding or the suppliers' inability to fill orders. The approximate cost of a 1 ml ampoule of naloxone in the US is estimated to be significantly higher than in most other countries. Take-home naloxone programs for people who use opioids is under way in many North American cities. CDC estimates that the US programs for drug users and their caregivers prescribing take-home doses of naloxone and training on its use prevented 10,000 opioid overdose deaths by 2014. In Australia, as of February 1, 2016, some forms of naloxone are available "over the counter" in pharmacies without a prescription. It comes in single-use filled syringe similar to law enforcement kits. A single dose costs AU$20; for those with a prescription, five doses can bought for AU$40, amounting to a rate of eight dollars per dose (2019). In Alberta, in addition to pharmacy distribution, take-home naloxone kits are available and commonly distributed in most drug treatment or rehabilitation centres.Naloxone kits now available at drug stores as province battles fentanyl crisis - Injection drug can temporarily reverse overdoses. Retrieved 29 February 2016. In Europe, take home naloxone pilots were launched in the Channel Islands and in Berlin in the late 1990s. In 2008 the Welsh Assembly government announced its intention to establish demonstration sites for take-home naloxone, and in 2010 Scotland instituted a national naloxone program. Inspired by North American and European efforts, non-governmental organizations running programs to train drug users as overdose responders and supply them with naloxone are now operational in Russia, Ukraine, Georgia, Kazakhstan, Tajikistan, Afghanistan, China, Vietnam, and Thailand. Noting the high risk of overdose among people with HIV who inject drugs, international HIV donors including the President's Emergency Plan for AIDS Relief, the The Global Fund to Fight AIDS, Tuberculosis and Malaria, Global Fund to Fight AIDS, Tuberculosis and Malaria, and the Open Society Foundations, have supported the purchase and distribution of naloxone to those at risk in low- and middle income countries. In 2017, Next Harm Reduction in New York State began distributing naloxone and other harm reduction supplies by mail to those in the US unable to get them locally. In 2018, a maker of naloxone announced it would provide a free kit including two doses of the nasal spray, as well as educational materials, to each of the 16,568 public libraries and 2,700 YMCAs in the U.S.
Media
The 2013 documentary film ''Reach for Me: Fighting to End the American Drug Overdose Epidemic'' interviews people involved in naloxone programs aiming to make naloxone available to opioid users and people with chronic pain.Criticism
Some political commentators, law enforcement workers, and addiction specialists have argued naloxone enables opioid addiction and worsens the crisis. Some police officers report reviving the same addict multiple times and that the availability of naloxone have allowed some addicts to push their use over the edge. Radio host Lars Larson claimed that naloxone only works for an hour, and if a person does not receive stabilizing medical help in that time, the addict just overdoses again. Other critics have noted Narcan nasal spray's American manufacturer views colleges, schools, libraries, and community centers as "untapped markets" and a "growth opportunity." Narcan's manufacturer also charges $150 for the nasal spray and aggressively sues competitors looking to market a cheaper unauthorized generic version of the drug. The public relations effort to raise awareness of naloxone and promote policies such as bulk purchases by police departments obviously increases sales.See also
* Buprenorphine/naloxone * Oxycodone/naloxone * Naloxazone, the hydrazone analogReferences
Further reading
*External links
* * * * * {{Emergency medicine Allylamines Antidotes Chemical substances for emergency medicine 4,5-Epoxymorphinans GABAA receptor negative allosteric modulators Kappa-opioid receptor antagonists Ketones Mu-opioid receptor antagonists Phenol ethers Sigma antagonists Tertiary alcohols Wikipedia medicine articles ready to translate World Health Organization essential medicines