Nuclear Reactor Heat Removal on:
[Wikipedia]
[Google]
[Amazon]
The removal of heat from nuclear reactors is an essential step in the generation of energy from
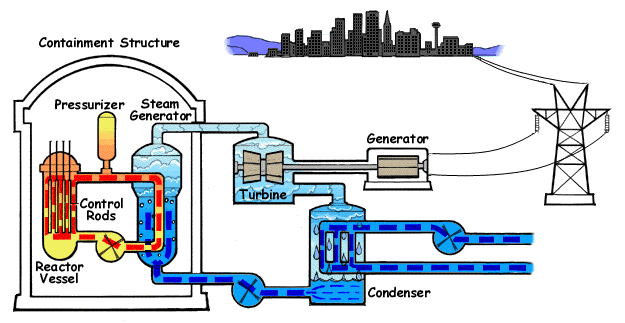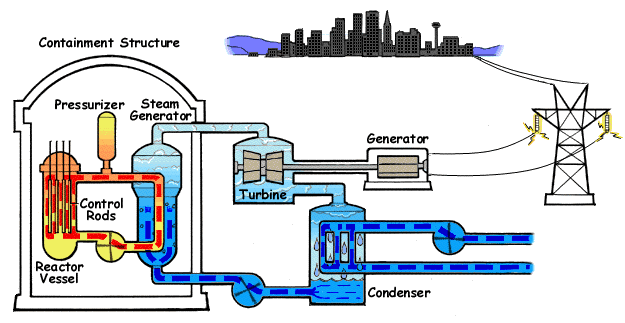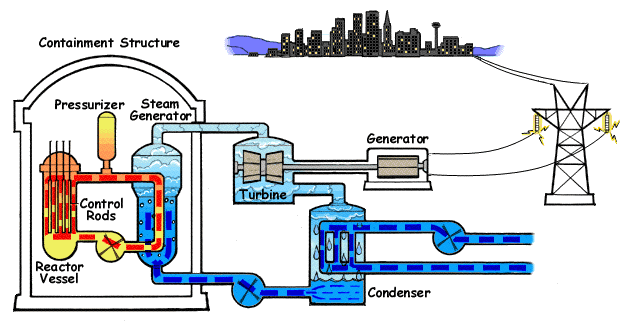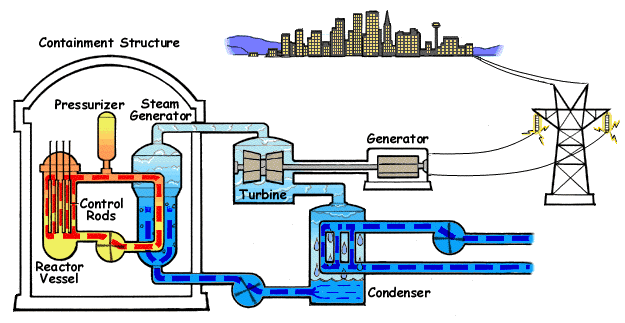 Increasing the flow of heat, reducing the agent flow and lowering the pressure can lead to increased temperature of the cooled surface. If the temperature of the fluid in the channel section that we consider is lower than the boiling temperature under local pressure conditions, the
Increasing the flow of heat, reducing the agent flow and lowering the pressure can lead to increased temperature of the cooled surface. If the temperature of the fluid in the channel section that we consider is lower than the boiling temperature under local pressure conditions, the
nuclear reactions
In nuclear physics and nuclear chemistry, a nuclear reaction is a process in which two nuclei, or a nucleus and an external subatomic particle, collide to produce one or more new nuclides. Thus, a nuclear reaction must cause a transformation o ...
. In nuclear engineering
Nuclear engineering is the branch of engineering concerned with the application of breaking down atomic nuclei ( fission) or of combining atomic nuclei (fusion), or with the application of other sub-atomic processes based on the principles of n ...
there are a number of empirical or semi-empirical relations used for quantifying the process of removing heat from a nuclear reactor core so that the reactor operates in the projected temperature interval that depends on the materials used in the construction of the reactor. The effectiveness of removal of heat from the reactor core depends on many factors, including the cooling agents used and the type of reactor. Common liquid coolants for nuclear reactors include: deionized water
Purified water is water that has been mechanically filtered or processed to remove impurities and make it suitable for use. Distilled water was, formerly, the most common form of purified water, but, in recent years, water is more frequently puri ...
(with boric acid
Boric acid, more specifically orthoboric acid, is a compound of boron, oxygen, and hydrogen with formula . It may also be called hydrogen borate or boracic acid. It is usually encountered as colorless crystals or a white powder, that dissolves ...
as a chemical shim
In applications such as nuclear reactors, a neutron poison (also called a neutron absorber or a nuclear poison) is a substance with a large Neutron cross section, neutron absorption cross-section. In such applications, absorbing neutrons is norma ...
during early burnup
In nuclear power technology, burnup (also known as fuel utilization) is a measure of how much energy is extracted from a primary nuclear fuel source. It is measured as the fraction of fuel atoms that underwent fission in %FIMA (fissions per init ...
), heavy water, the lighter alkaline metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
s (such as sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable iso ...
and lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid el ...
), lead
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cu ...
or lead-based eutectic alloys like lead-bismuth, and NaK
In data networking, telecommunications, and computer buses, an acknowledgment (ACK) is a signal that is passed between communicating processes, computers, or devices to signify acknowledgment, or receipt of message, as part of a communicatio ...
, a eutectic alloy of sodium and potassium. Gas cooled reactor
A gas-cooled reactor (GCR) is a nuclear reactor that uses graphite as a neutron moderator and a gas (carbon dioxide or helium in extant designs) as coolant. Although there are many other types of reactor cooled by gas, the terms ''GCR'' and to a l ...
s operate with coolants like carbon dioxide, helium or nitrogen but some very low powered research reactor
Research reactors are nuclear fission-based nuclear reactors that serve primarily as a neutron source. They are also called non-power reactors, in contrast to power reactors that are used for electricity production, heat generation, or maritim ...
s have even been air-cooled with Chicago Pile 1
Chicago Pile-1 (CP-1) was the world's first artificial nuclear reactor. On 2 December 1942, the first human-made self-sustaining nuclear chain reaction was initiated in CP-1, during an experiment led by Enrico Fermi. The secret development of t ...
relying on natural convection of the surrounding air to remove the negligible thermal power output. There is ongoing research into using supercritical fluid
A supercritical fluid (SCF) is any substance at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist, but below the pressure required to compress it into a solid. It can effuse through porous so ...
s as reactor coolants but thus far neither the supercritical water reactor
The supercritical water reactor (SCWR) is a concept Generation IV reactor, designed as a light water reactor (LWR) that operates at supercritical pressure (i.e. greater than 22.1 MPa). The term ''critical'' in this context refers to the c ...
nor a reactor cooled with supercritical Carbon Dioxide
Supercritical carbon dioxide (s) is a fluid state of carbon dioxide where it is held at or above its critical temperature and critical pressure.
Carbon dioxide usually behaves as a gas in air at standard temperature and pressure (STP), or as ...
nor any other kind of supercritical-fluid-cooled reactor has ever been built.
Theoretical framework
The thermal energy produced innuclear fuel
Nuclear fuel is material used in nuclear power stations to produce heat to power turbines. Heat is created when nuclear fuel undergoes nuclear fission.
Most nuclear fuels contain heavy fissile actinide elements that are capable of undergoing ...
comes mainly from the kinetic energy of fission fragments. Therefore, the heat generated per volume unit is proportional to the fraction of nuclear fissionable fuel burned in the unit of time:
where represents the number of atoms in a cubic meter of fuel, a is the amount of energy released in the fuel in each fission reaction (~181 MeV), is the neutronic flux, and is the effective section of the fission.
The total heat produced in the nuclear reactor
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat from nu ...
is:
where is the mean neutronic flux and V is the fuel volume (normally measured in ).
Recovery of this amount of heat is achieved by using cooling fluids whose temperature at the entrance to the reactor channel will increase with the distance traveled in the channel. The thermal balance of the channel is expressed by the relationship:
where is the flow rate of the cooling agent, is the specific heat at constant pressure, is the increase in the temperature of the fluid after passing a distance in the channel, is the heat generated per unit volume of the fuel, is the fuel cell radius and is the number of channel bars.
Under these conditions, the temperature of the cooling agent at distance z travelled into the cooling channel inside nuclear reactor is obtained by integrating the previous equation:
The difference between the temperature of the outer surface of the tube-channel and the temperature of the fluid is obtained from the relationship:
where is the local heat flow on the casing - cooler contact surface unit and is the heat transfer agent casing-cooling agent.
The heat discharge from the PWR and PHWR
A pressurized heavy-water reactor (PHWR) is a nuclear reactor that uses heavy water ( deuterium oxide D2O) as its coolant and neutron moderator. PHWRs frequently use natural uranium as fuel, but sometimes also use very low enriched uranium. The ...
reactors is made by pressurized water under forced convection
Convection is single or multiphase fluid flow that occurs spontaneously due to the combined effects of material property heterogeneity and body forces on a fluid, most commonly density and gravity (see buoyancy). When the cause of the convec ...
. The general expression for determining the transfer coefficient is given by the Dittus - Boelter equation:
where is Nusselt's number ( , is the heat transfer coefficient, is the equivalent diameter, is the thermal conductivity of the fluid); is a constant (=0.023); is the number of Reynolds ( ) is the average velocity of the fluid in the section considered, is the density of the fluid and is its dynamic viscosity
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water.
Viscosity quantifies the inter ...
); is the number of Prandtl ().
If the flow of the fluid is made under conditions of a great difference between its temperature and the contact surface, the transfer coefficient is determined from the relationship:
where is the dynamic viscosity
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water.
Viscosity quantifies the inter ...
of the coolant
A coolant is a substance, typically liquid, that is used to reduce or regulate the temperature of a system. An ideal coolant has high thermal capacity, low viscosity, is low-cost, non-toxic, chemically inert and neither causes nor promotes corrosio ...
at the temperature of the adhering fluid film at the surface of the casing. The relation presented above is valid in the case of a long channel with , where is the length of the channel.
The transfer coefficient for cooling the pipes by natural convection
Convection is single or multiphase fluid flow that occurs spontaneously due to the combined effects of material property heterogeneity and body forces on a fluid, most commonly density and gravity (see buoyancy). When the cause of the convec ...
is obtained from:
where is the Grashof number given by the expression:
We use the notation for the volume expansion coefficient of the fluid, is the gravitational acceleration and is the difference between the average wall temperatures of the casing and the cooling agent.
In boiling water cooled reactors (BWR) and partly in pressure water cooled reactors ( PWR and PHWR
A pressurized heavy-water reactor (PHWR) is a nuclear reactor that uses heavy water ( deuterium oxide D2O) as its coolant and neutron moderator. PHWRs frequently use natural uranium as fuel, but sometimes also use very low enriched uranium. The ...
) the heat transfer is made with a vapor phase in the cooling medium, which is why this type of heat transfer is called heat transfer in a biphasic system. This allows obtaining much higher transfer coefficients than the one-phase heat transfer described in the Dittus-Boelter equation.
 Increasing the flow of heat, reducing the agent flow and lowering the pressure can lead to increased temperature of the cooled surface. If the temperature of the fluid in the channel section that we consider is lower than the boiling temperature under local pressure conditions, the
Increasing the flow of heat, reducing the agent flow and lowering the pressure can lead to increased temperature of the cooled surface. If the temperature of the fluid in the channel section that we consider is lower than the boiling temperature under local pressure conditions, the vaporization
Vaporization (or vaporisation) of an element or compound is a phase transition from the liquid phase to vapor. There are two types of vaporization: evaporation and boiling. Evaporation is a surface phenomenon, whereas boiling is a bulk phenomen ...
is limited to the immediate vicinity of the surface and in this case the boiling is called submerged boiling. There is no proportionality between the heat flow and the difference between the surface temperature and the coolant temperature that allows the definition of a heat transfer coefficient similar to the one-phase case. In this situation we can use the equation of Jens and Lottes, which establishes a connection between the difference between the surface temperature and the boiling temperature of the cooling agent under local pressure conditions below the thermal flux :
where and
If the temperature of the fluid in the channel section considered is slightly higher than the boiling temperature under local pressure conditions, the heat transfer is by boiling with nucleation, forming vapor bubbles trained by the cooling agent (that becomes biphasic throughout its entire volume). However, the vapor content is relatively small and the continuous phase remains the liquid phase. The vapor content of the PHW-CANDU reactor is about 0.03-0.04 kg steam / kg of agent, thus increasing the amount of heat transported by the unit mass of agent by over 10%. If the cooled surface temperature far exceeds the boiling temperature of the cooling agent in the channel section, the vapor content of the agent increases considerably, the continuous phase becoming the vapor phase and the liquid phase becoming only a suspension between vapors. The cooled surface remains covered with a liquid film which still provides a very high heat transfer coefficient, at BWR compared to at PWR. The film of liquid is continuously fed with drops from the agent suspension
Suspension or suspended may refer to:
Science and engineering
* Suspension (topology), in mathematics
* Suspension (dynamical systems), in mathematics
* Suspension of a ring, in mathematics
* Suspension (chemistry), small solid particles suspend ...
.
A further increase in surface temperature leads to a temporary interruption of continuity of the liquid film adhering to the cooled surface. Watering of the surface continues, however, by the drops of liquid in the suspension
Suspension or suspended may refer to:
Science and engineering
* Suspension (topology), in mathematics
* Suspension (dynamical systems), in mathematics
* Suspension of a ring, in mathematics
* Suspension (chemistry), small solid particles suspend ...
that are present in the cooling agent as long as the heat flow remains below a value that depends on local conditions (value that is called critical flux). Over this flux there is a thermal transfer crisis characterized by a sudden decrease in the transfer coefficient due to the presence of only one-phase transfer. The heat transfer coefficient in the pre-crisis period can be determined from the relationship:
where
In these formulas the following notations were made: is pressure losses for the two phases (water and vapors), ( - the thermal flux, - the enthalpy of the biphasic liquid-gaseous mixture). The heat transfer coefficient during the crisis is related to the critical heat flow through a linear relationship, of the equation type that was presented before:
Where is the temperature of the surface in thermal transfer crisis, and is the temperature of the vapor at saturation.
The critical flow is obtained by using the Kutateladze's formula:
where (J/kg)is the latent heat of vaporization, and are density of the liquid and saturation vapor, is the superficial tension in N / m and is the gravitational acceleration. The heat transfer to the gas-cooled reactors is carried out by forced convection. For a gaseous thermal agent, the heat transfer coefficient can be deduced from a relation of the type Dittus-Boelter, but taking into account, for the intervening sizes, the values corresponding to the average temperature of the fluid film denoted by the index :
which differs in the use of water by a slightly lower value of the coefficient a.
Forced flow relationships established for fluids are also not valid for liquid metals. The coefficient of heat transfer for circular pipelines with constant heat flux, where the heat evacuation is achieved by the turbulent flow
In fluid dynamics, turbulence or turbulent flow is fluid motion characterized by chaotic changes in pressure and flow velocity. It is in contrast to a laminar flow, which occurs when a fluid flows in parallel layers, with no disruption between ...
of the molten metals, can be estimated with a relation of the type:
where is the number of Peclet ().
Examples of heat evacuation hydrodynamic parameters
For exemplification of the above formulas the hydrodynamic parameters of some types of reactors can be found in the following table: G1 and EL-4 are reactors that were built in France, whileVVER-440
The water-water energetic reactor (WWER), or VVER (from russian: водо-водяной энергетический реактор; transliterates as ; ''water-water power reactor'') is a series of pressurized water reactor designs originally de ...
is a reactor that has been constructed in the Soviet Union.
References
{{Reflist Nuclear reactors Nuclear power Cooling technology