NaCl on:
[Wikipedia]
[Google]
[Amazon]
Sodium chloride , commonly known as
2 NaCl + 2 H2O -> lectrolysisCl2 + H2 + 2 NaOH
This electrolysis is conducted in either a mercury cell, a diaphragm cell, or a membrane cell. Each of those uses a different method to separate the chlorine from the sodium hydroxide. Other technologies are under development due to the high energy consumption of the electrolysis, whereby small improvements in the efficiency can have large economic paybacks. Some applications of chlorine include PVC
 The second major application of salt is for
The second major application of salt is for  Salt for de-icing in the United Kingdom predominantly comes from a single mine in
Salt for de-icing in the United Kingdom predominantly comes from a single mine in
 Sodium chloride is the principal extinguishing agent in fire extinguishers (Met-L-X, Super D) used on combustible metal fires such as magnesium, potassium, sodium, and NaK alloys (Class D).
Sodium chloride is the principal extinguishing agent in fire extinguishers (Met-L-X, Super D) used on combustible metal fires such as magnesium, potassium, sodium, and NaK alloys (Class D).
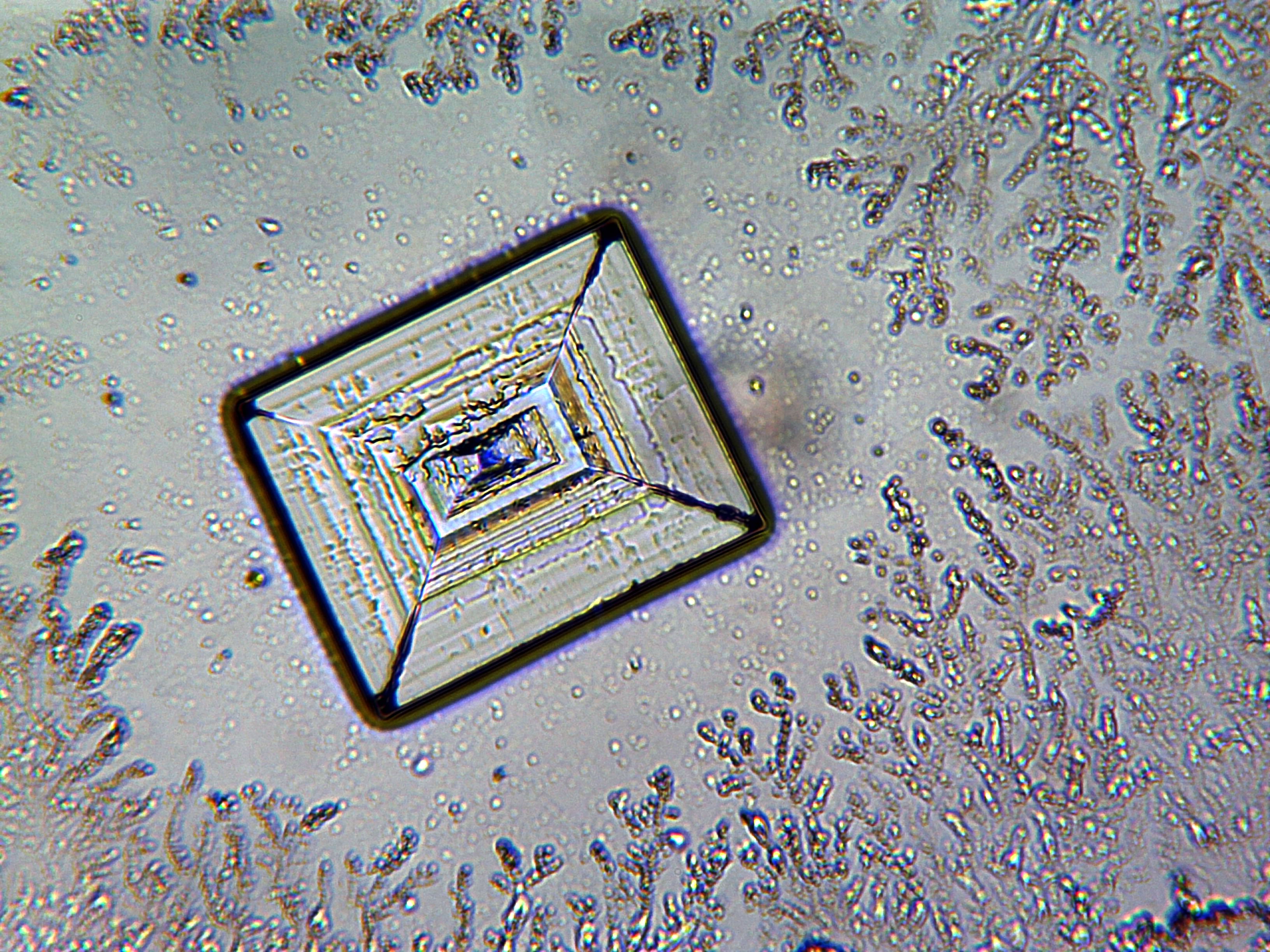
 In solid sodium chloride, each ion is surrounded by six ions of the opposite charge as expected on electrostatic grounds. The surrounding ions are located at the vertices of a regular
In solid sodium chloride, each ion is surrounded by six ions of the opposite charge as expected on electrostatic grounds. The surrounding ions are located at the vertices of a regular
 When dissolved in water, the sodium chloride framework disintegrates as the Na+ and Cl− ions become surrounded by polar water molecules. These solutions consist of
When dissolved in water, the sodium chloride framework disintegrates as the Na+ and Cl− ions become surrounded by polar water molecules. These solutions consist of
"Salt"
in ''U.S. Geological Survey, 2008 Minerals Yearbook'' In 2017, world production was estimated at 280 millionSalt
U.S. Geological Survey Salt is also a byproduct of
File:Salt mine 0096.jpg, Modern rock salt mine near Mount Morris, New York,
Salt
surface tensions
an
densities, molarities and molalities
of aqueous NaCl (and other salts)
{{DEFAULTSORT:Sodium Chloride Alkali metal chlorides Chlorides Deliquescent substances Household chemicals Metal halides Sodium compounds Sodium minerals Rock salt crystal structure Ophthalmology drugs
salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quantitie ...
(although sea salt
Sea salt is salt that is produced by the evaporation of seawater. It is used as a seasoning in foods, cooking, cosmetics and for preserving food. It is also called bay salt, solar salt, or simply salt. Like mined rock salt, production of sea sa ...
also contains other chemical salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quantitie ...
s), is an ionic compound with the chemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
NaCl, representing a 1:1 ratio of sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable iso ...
and chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride salts ...
ions. With molar mass
In chemistry, the molar mass of a chemical compound is defined as the mass of a sample of that compound divided by the amount of substance which is the number of moles in that sample, measured in moles. The molar mass is a bulk, not molecular, p ...
es of 22.99 and 35.45 g/mol respectively, 100 g of NaCl contains 39.34 g Na and 60.66 g Cl. Sodium chloride is the salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quantitie ...
most responsible for the salinity
Salinity () is the saltiness or amount of salt dissolved in a body of water, called saline water (see also soil salinity). It is usually measured in g/L or g/kg (grams of salt per liter/kilogram of water; the latter is dimensionless and equal ...
of seawater
Seawater, or salt water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has appro ...
and of the extracellular fluid
In cell biology, extracellular fluid (ECF) denotes all body fluid outside the cells of any multicellular organism. Total body water in healthy adults is about 60% (range 45 to 75%) of total body weight; women and the obese typically have a lower ...
of many multicellular organism
A multicellular organism is an organism that consists of more than one cell, in contrast to unicellular organism.
All species of animals, land plants and most fungi are multicellular, as are many algae, whereas a few organisms are partially uni- ...
s. In its edible form, salt (also known as ''table salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quantitie ...
'') is commonly used as a condiment
A condiment is a preparation that is added to food, typically after cooking, to impart a specific Flavoring, flavor, to enhance the flavor, or to complement the dish. A table condiment or table sauce is more specifically a condiment that is serv ...
and food preservative
Food preservation includes processes that make food more resistant to microorganism growth and slow the oxidation of fats. This slows down the decomposition and rancidification process. Food preservation may also include processes that inhibit ...
. Large quantities of sodium chloride are used in many industrial processes, and it is a major source of sodium and chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
compounds used as feedstock
A raw material, also known as a feedstock, unprocessed material, or primary commodity, is a basic material that is used to produce goods, finished goods, energy, or intermediate materials that are feedstock for future finished products. As feedst ...
s for further chemical syntheses. Another major application of sodium chloride is de-icing of roadways in sub-freezing weather.
Uses
In addition to the familiar domestic uses of salt, more dominant applications of the approximately 250 million tonnes per year production (2008 data) include chemicals and de-icing.Westphal, Gisbert ''et al.'' (2002) "Sodium Chloride" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim .Chemical functions
Salt is used, directly or indirectly, in the production of many chemicals, which consume most of the world's production.Chlor-alkali industry
It is the starting point for thechloralkali process
The chloralkali process (also chlor-alkali and chlor alkali) is an industrial process for the electrolysis of sodium chloride (NaCl) solutions. It is the technology used to produce chlorine and sodium hydroxide (caustic soda), which are comm ...
, the industrial process to produce chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
and sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and alkali ...
, according to the chemical equation
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and chemical formulas. The reactant entities are given on the left-hand side and the product entities on the right-hand side with a plus sign between ...
:thermoplastic
A thermoplastic, or thermosoft plastic, is any plastic polymer material that becomes pliable or moldable at a certain elevated temperature and solidifies upon cooling.
Most thermoplastics have a high molecular weight. The polymer chains associate ...
s production, disinfectants, and solvents.
Sodium hydroxide is extensively used in many different industries enabling production of paper, soap, and aluminium etc.
Soda-ash industry
Sodium chloride is used in theSolvay process
The Solvay process or ammonia-soda process is the major industrial process for the production of sodium carbonate (soda ash, Na2CO3). The ammonia-soda process was developed into its modern form by the Belgian chemist Ernest Solvay during the 1860s. ...
to produce sodium carbonate
Sodium carbonate, , (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield moderately alkaline solutions ...
and calcium chloride
Calcium chloride is an inorganic compound, a salt with the chemical formula . It is a white crystalline solid at room temperature, and it is highly soluble in water. It can be created by neutralising hydrochloric acid with calcium hydroxide.
Ca ...
. Sodium carbonate, in turn, is used to produce glass
Glass is a non-crystalline, often transparent, amorphous solid that has widespread practical, technological, and decorative use in, for example, window panes, tableware, and optics. Glass is most often formed by rapid cooling (quenching) of ...
, sodium bicarbonate
Sodium bicarbonate (IUPAC name: sodium hydrogencarbonate), commonly known as baking soda or bicarbonate of soda, is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cation ( Na+) and a bicarbonate anion ( HCO3−) ...
, and dyes, as well as a myriad of other chemicals. In the Mannheim process
The Mannheim process is an industrial process for the production of hydrogen chloride and sodium sulfate from sulfuric acid and sodium chloride. The Mannheim furnace is also used to produce potassium sulfate from potassium chloride. The Mannheim ...
, sodium chloride is used for the production of sodium sulfate
Sodium sulfate (also known as sodium sulphate or sulfate of soda) is the inorganic compound with formula Na2SO4 as well as several related hydrates. All forms are white solids that are highly soluble in water. With an annual production of 6 milli ...
and hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
.
Standard
Sodium chloride has an international standard that is created byASTM International
ASTM International, formerly known as American Society for Testing and Materials, is an international standards organization that develops and publishes voluntary consensus technical standards for a wide range of materials, products, systems, ...
. The standard is named ASTM E534-13 and is the standard test methods for chemical analysis of sodium chloride. These methods listed provide procedures for analyzing sodium chloride to determine whether it is suitable for its intended use and application.
Miscellaneous industrial uses
Sodium chloride is heavily used, so even relatively minor applications can consume massive quantities. Inoil
An oil is any nonpolar chemical substance that is composed primarily of hydrocarbons and is hydrophobic (does not mix with water) & lipophilic (mixes with other oils). Oils are usually flammable and surface active. Most oils are unsaturated ...
and gas
Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma).
A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), or ...
exploration, salt is an important component of drilling fluids in well drilling. It is used to flocculate
Flocculation, in the field of chemistry, is a process by which colloidal particles come out of suspension to sediment under the form of floc or flake, either spontaneously or due to the addition of a clarifying agent. The action differs from p ...
and increase the density
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' can also be used. Mathematical ...
of the drilling fluid to overcome high downwell gas pressures. Whenever a drill hits a salt formation, salt is added to the drilling fluid to saturate the solution in order to minimize the dissolution within the salt stratum. Salt is also used to increase the curing of concrete in cemented casings.
In textiles and dyeing, salt is used as a brine rinse to separate organic contaminants, to promote "salting out" of dyestuff precipitates, and to blend with concentrated dyes to standardize them. One of its main roles is to provide the positive ion charge to promote the absorption of negatively charged ions of dyes.
It is also used in processing aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. I ...
, beryllium
Beryllium is a chemical element with the symbol Be and atomic number 4. It is a steel-gray, strong, lightweight and brittle alkaline earth metal. It is a divalent element that occurs naturally only in combination with other elements to form mi ...
, copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkis ...
, steel
Steel is an alloy made up of iron with added carbon to improve its strength and fracture resistance compared to other forms of iron. Many other elements may be present or added. Stainless steels that are corrosion- and oxidation-resistant ty ...
and vanadium
Vanadium is a chemical element with the symbol V and atomic number 23. It is a hard, silvery-grey, malleable transition metal. The elemental metal is rarely found in nature, but once isolated artificially, the formation of an oxide layer ( pas ...
. In the pulp and paper industry
The pulp and paper industry comprises companies that use wood as raw material and produce pulp, paper, paperboard and other cellulose-based products.
Manufacturing process
The pulp is fed to a paper machine where it is formed as a paper web an ...
, salt is used to bleach wood pulp. It also is used to make sodium chlorate
Sodium chlorate is an inorganic compound with the chemical formula Na ClO3. It is a white crystalline powder that is readily soluble in water. It is hygroscopic. It decomposes above 300 °C to release oxygen and leaves sodium chloride. Sever ...
, which is added along with sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formu ...
and water to manufacture chlorine dioxide
Chlorine dioxide is a chemical compound with the formula ClO2 that exists as yellowish-green gas above 11 °C, a reddish-brown liquid between 11 °C and −59 °C, and as bright orange crystals below −59 °C. It is usually ...
, an excellent oxygen-based bleach
Bleach is the generic name for any chemical product that is used industrially or domestically to remove color (whitening) from a fabric or fiber or to clean or to remove stains in a process called bleaching. It often refers specifically, to ...
ing chemical. The chlorine dioxide process, which originated in Germany after World War I, is becoming more popular because of environmental pressures to reduce or eliminate chlorinated bleaching compounds. In tanning and leather treatment, salt is added to animal hides to inhibit microbial activity on the underside of the hides and to attract moisture back into the hides.
In rubber manufacture, salt is used to make buna, neoprene
Neoprene (also polychloroprene) is a family of synthetic rubbers that are produced by polymerization of chloroprene.Werner Obrecht, Jean-Pierre Lambert, Michael Happ, Christiane Oppenheimer-Stix, John Dunn and Ralf Krüger "Rubber, 4. Emulsion R ...
and white rubber types. Salt brine and sulfuric acid are used to coagulate an emulsified latex
Latex is an emulsion (stable dispersion) of polymer microparticles in water. Latexes are found in nature, but synthetic latexes are common as well.
In nature, latex is found as a milky fluid found in 10% of all flowering plants (angiosperms ...
made from chlorinated butadiene
1,3-Butadiene () is the organic compound with the formula (CH2=CH)2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two viny ...
.
Salt also is added to secure the soil and to provide firmness to the foundation on which highways are built. The salt acts to minimize the effects of shifting caused in the subsurface by changes in humidity and traffic load.
Sodium chloride is sometimes used as a cheap and safe desiccant because of its hygroscopic
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substance ...
properties, making salting an effective method of food preservation
Food preservation includes processes that make food more resistant to microorganism growth and slow the oxidation of fats. This slows down the decomposition and rancidification process. Food preservation may also include processes that inhibit ...
historically; the salt draws water out of bacteria through osmotic pressure
Osmotic pressure is the minimum pressure which needs to be applied to a solution to prevent the inward flow of its pure solvent across a semipermeable membrane.
It is also defined as the measure of the tendency of a solution to take in a pure ...
, keeping it from reproducing, a major source of food spoilage. Even though more effective desiccants are available, few are safe for humans to ingest.
Water softening
Hard water
Hard water is water that has high mineral content (in contrast with "soft water"). Hard water is formed when water percolates through deposits of limestone, chalk or gypsum, which are largely made up of calcium and magnesium carbonates, bicarbo ...
contains calcium and magnesium ions that interfere with action of soap
Soap is a salt of a fatty acid used in a variety of cleansing and lubricating products. In a domestic setting, soaps are surfactants usually used for washing, bathing, and other types of housekeeping. In industrial settings, soaps are use ...
and contribute to the buildup of a scale or film of alkaline mineral deposits in household and industrial equipment and pipes. Commercial and residential water-softening units use ion-exchange resin
An ion-exchange resin or ion-exchange polymer is a resin or polymer that acts as a medium for ion exchange. It is an insoluble matrix (or support structure) normally in the form of small (0.25–1.43 mm radius) microbeads, usually white or ye ...
s to remove ions that cause the hardness. These resins are generated and regenerated using sodium chloride.
Road salt
 The second major application of salt is for
The second major application of salt is for de-icing
Deicing is the process of removing snow, ice or frost from a surface. Anti-icing is the application of chemicals that not only deice but also remain on a surface and continue to delay the reformation of ice for a certain period of time, or prev ...
and anti-icing of roads, both in grit bin
A grit bin, salt bin or sand bin is an item of street furniture, commonly found in countries where freezing temperatures and snowfall occur, which holds a mixture of salt and grit that is spread over roads if they have snow or ice on them. Sprea ...
s and spread by winter service vehicle
A winter service vehicle (WSV), or snow removal vehicle, is a vehicle specially designed or adapted to clear thoroughfares of ice and snow. Winter service vehicles are usually based on a dump truck chassis, with adaptations allowing them to carr ...
s. In anticipation of snowfall, roads are optimally "anti-iced" with brine (concentrated solution
Solution may refer to:
* Solution (chemistry), a mixture where one substance is dissolved in another
* Solution (equation), in mathematics
** Numerical solution, in numerical analysis, approximate solutions within specified error bounds
* Soluti ...
of salt in water), which prevents bonding between the snow-ice and the road surface. This procedure obviates the heavy use of salt after the snowfall. For de-icing, mixtures of brine and salt are used, sometimes with additional agents such as calcium chloride
Calcium chloride is an inorganic compound, a salt with the chemical formula . It is a white crystalline solid at room temperature, and it is highly soluble in water. It can be created by neutralising hydrochloric acid with calcium hydroxide.
Ca ...
and/or magnesium chloride
Magnesium chloride is the family of inorganic compounds with the formula , where x can range from 0 to 12. These salts are colorless or white solids that are highly soluble in water. These compounds and their solutions, both of which occur in natu ...
. The use of salt or brine becomes ineffective below .
 Salt for de-icing in the United Kingdom predominantly comes from a single mine in
Salt for de-icing in the United Kingdom predominantly comes from a single mine in Winsford
Winsford is a town and civil parish in the unitary authority of Cheshire West and Chester and the ceremonial county of Cheshire, England, on the River Weaver south of Northwich and west of Middlewich. It grew around the salt mining industry ...
in Cheshire
Cheshire ( ) is a ceremonial and historic county in North West England, bordered by Wales to the west, Merseyside and Greater Manchester to the north, Derbyshire to the east, and Staffordshire and Shropshire to the south. Cheshire's county t ...
. Prior to distribution it is mixed with <100 ppm of sodium ferrocyanide
Sodium ferrocyanide is the sodium salt of the coordination compound of formula e(CN)6sup>4−. In its hydrous form, Na4Fe(CN)6 ( sodium ferrocyanide decahydrate), it is sometimes known as yellow prussiate of soda. It is a yellow crystalline ...
as an anti-caking agent, which enables rock salt to flow freely out of the gritting vehicles despite being stockpiled prior to use. In recent years this additive has also been used in table salt. Other additives had been used in road salt to reduce the total costs. For example, in the US, a byproduct carbohydrate solution from sugar-beet processing was mixed with rock salt and adhered to road surfaces about 40% better than loose rock salt alone. Because it stayed on the road longer, the treatment did not have to be repeated several times, saving time and money.
In the technical terms of physical chemistry, the minimum freezing point of a water-salt mixture is for 23.31 wt% of salt. Freezing near this concentration is however so slow that the eutectic point
A eutectic system or eutectic mixture ( ) is a homogeneous mixture that has a melting point lower than those of the constituents. The lowest possible melting point over all of the mixing ratios of the constituents is called the ''eutectic tempe ...
of can be reached with about 25 wt% of salt.
Environmental effects
Road salt ends up in fresh-water bodies and could harm aquatic plants and animals by disrupting theirosmoregulation
Osmoregulation is the active regulation of the osmotic pressure of an organism's body fluids, detected by osmoreceptors, to maintain the homeostasis of the organism's water content; that is, it maintains the fluid balance and the concentration o ...
ability. The omnipresence of salt poses a problem in any coastal coating application, as trapped salts cause great problems in adhesion. Naval authorities and ship builders monitor the salt concentrations on surfaces during construction. Maximal salt concentrations on surfaces are dependent on the authority and application. The IMO regulation is mostly used and sets salt levels to a maximum of 50 mg/m2 soluble salts measured as sodium chloride. These measurements are done by means of a Bresle test
The Bresle method is used to determine concentration of soluble salts on metal surfaces prior to coating application, such as painting. These salts can cause serious adhesion problems after time.
Importance
Salt is in coastal areas. It can be ta ...
. Salinization (increasing salinity, aka ''freshwater salinization
Freshwater salinization is the process of salty runoff contaminating freshwater ecosystems, which can harm aquatic species in certain quantities and Drinking water, contaminate drinking water. It is often measured by the increased amount of dissolv ...
syndrome'') and subsequent increased metal leaching is an ongoing problem throughout North America and European fresh waterways.
In highway de-icing, salt has been associated with corrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engine ...
of bridge decks, motor vehicles, reinforcement bar and wire, and unprotected steel structures used in road construction. Surface runoff
Surface runoff (also known as overland flow) is the flow of water occurring on the ground surface when excess rainwater, stormwater, meltwater, or other sources, can no longer sufficiently rapidly infiltrate in the soil. This can occur when th ...
, vehicle spraying, and windblown actions also affect soil, roadside vegetation, and local surface water and groundwater supplies. Although evidence of environmental loading of salt has been found during peak usage, the spring rains and thaws usually dilute the concentrations of sodium in the area where salt was applied. A 2009 study found that approximately 70% of the road salt being applied in the Minneapolis-St Paul metro area is retained in the local watershed.
Substitution
Some agencies are substitutingbeer
Beer is one of the oldest and the most widely consumed type of alcoholic drink in the world, and the third most popular drink overall after water and tea. It is produced by the brewing and fermentation of starches, mainly derived from ce ...
, molasses
Molasses () is a viscous substance resulting from refining sugarcane or sugar beets into sugar. Molasses varies in the amount of sugar, method of extraction and age of the plant. Sugarcane molasses is primarily used to sweeten and flavour foods ...
, and beet
The beetroot is the taproot portion of a beet plant, usually known in North America as beets while the vegetable is referred to as beetroot in British English, and also known as the table beet, garden beet, red beet, dinner beet or golden beet ...
juice instead of road salt
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g/ ...
. Airlines utilize more glycol
A diol is a chemical compound containing two hydroxyl groups ( groups). An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified.
The most common industrial diol is e ...
and sugar
Sugar is the generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. Simple sugars, also called monosaccharides, include glucose, fructose, and galactose. Compound sugars, also called disaccharides or double ...
rather than salt based solutions for de-icing
Deicing is the process of removing snow, ice or frost from a surface. Anti-icing is the application of chemicals that not only deice but also remain on a surface and continue to delay the reformation of ice for a certain period of time, or prev ...
.
Food industry and agriculture
Manymicroorganism
A microorganism, or microbe,, ''mikros'', "small") and ''organism'' from the el, ὀργανισμός, ''organismós'', "organism"). It is usually written as a single word but is sometimes hyphenated (''micro-organism''), especially in olde ...
s cannot live in a salty environment: water is drawn out of their cells by osmosis
Osmosis (, ) is the spontaneous net movement or diffusion of solvent molecules through a selectively-permeable membrane from a region of high water potential (region of lower solute concentration) to a region of low water potential (region of ...
. For this reason salt is used to preserve
The word preserve may refer to:
Common uses
* Fruit preserves, a type of sweet spread or condiment
* Nature reserve, an area of importance for wildlife, flora, fauna or other special interest, usually protected
Arts, entertainment, and media
...
some foods, such as bacon, fish, or cabbage.
Salt is added to food, either by the food producer or by the consumer, as a flavor enhancer, preservative, binder, fermentation
Fermentation is a metabolic process that produces chemical changes in organic substrates through the action of enzymes. In biochemistry, it is narrowly defined as the extraction of energy from carbohydrates in the absence of oxygen. In food ...
-control additive, texture-control agent and color developer. The salt consumption in the food industry is subdivided, in descending order of consumption, into other food processing, meat packers, canning
Canning is a method of food preservation in which food is processed and sealed in an airtight container (jars like Mason jars, and steel and tin cans). Canning provides a shelf life that typically ranges from one to five years, although u ...
, baking, dairy and grain mill products. Salt is added to promote color development in bacon, ham and other processed meat products. As a preservative, salt inhibits the growth of bacteria. Salt acts as a binder in sausage
A sausage is a type of meat product usually made from ground meat—often pork, beef, or poultry—along with salt, spices and other flavourings. Other ingredients, such as grains or breadcrumbs may be included as fillers or extenders.
...
s to form a binding gel made up of meat, fat, and moisture. Salt also acts as a flavor enhancer and as a tenderizer.
In many dairy industries, salt is added to cheese as a color-, fermentation-, and texture-control agent. The dairy subsector includes companies that manufacture creamery butter, condensed and evaporated milk, frozen desserts, ice cream, natural and processed cheese, and specialty dairy products. In canning, salt is primarily added as a flavor enhancer and preservative
A preservative is a substance or a chemical that is added to products such as food products, beverages, pharmaceutical drugs, paints, biological samples, cosmetics, wood, and many other products to prevent decomposition by microbial growth or by ...
. It also is used as a carrier for other ingredients, dehydrating agent, enzyme inhibitor and tenderizer. In baking, salt is added to control the rate of fermentation in bread dough. It also is used to strengthen the gluten
Gluten is a structural protein naturally found in certain cereal grains. Although "gluten" often only refers to wheat proteins, in medical literature it refers to the combination of prolamin and glutelin proteins naturally occurring in all grain ...
(the elastic protein-water complex in certain doughs) and as a flavor enhancer, such as a topping on baked goods. The food-processing category also contains grain mill products. These products consist of milling flour and rice and manufacturing cereal breakfast food and blended or prepared flour. Salt is also used a seasoning agent, e.g. in potato chips, pretzel
A pretzel (), from German pronunciation, standard german: Breze(l) ( and French / Alsatian: ''Bretzel'') is a type of baked bread made from dough that is commonly shaped into a knot. The traditional pretzel shape is a distinctive symmetrical ...
s, cat and dog food.
Sodium chloride is used in veterinary medicine as emesis
Vomiting (also known as emesis and throwing up) is the involuntary, forceful expulsion of the contents of one's stomach through the mouth and sometimes the nose.
Vomiting can be the result of ailments like food poisoning, gastroenteriti ...
-causing agent. It is given as warm saturated solution. Emesis can also be caused by pharyngeal placement of small amount of plain salt or salt crystals.
Medicine
Sodium chloride is used together with water as one of the primary solutions forintravenous therapy
Intravenous therapy (abbreviated as IV therapy) is a medical technique that administers fluids, medications and nutrients directly into a person's vein. The intravenous route of administration is commonly used for rehydration or to provide nutri ...
. Nasal spray
Nasal sprays are used to deliver medications locally in the nasal cavities or systemically. They are used locally for conditions such as nasal congestion and allergic rhinitis. In some situations, the nasal delivery route is preferred for syste ...
often contains a saline solution.
Firefighting
 Sodium chloride is the principal extinguishing agent in fire extinguishers (Met-L-X, Super D) used on combustible metal fires such as magnesium, potassium, sodium, and NaK alloys (Class D).
Sodium chloride is the principal extinguishing agent in fire extinguishers (Met-L-X, Super D) used on combustible metal fires such as magnesium, potassium, sodium, and NaK alloys (Class D). Thermoplastic
A thermoplastic, or thermosoft plastic, is any plastic polymer material that becomes pliable or moldable at a certain elevated temperature and solidifies upon cooling.
Most thermoplastics have a high molecular weight. The polymer chains associate ...
powder is added to the mixture, along with waterproofing (metal stearates) and anti-caking materials (tricalcium phosphate) to form the extinguishing agent. When it is applied to the fire, the salt acts like a heat sink, dissipating heat from the fire, and also forms an oxygen-excluding crust to smother the fire. The plastic additive melts and helps the crust maintain its integrity until the burning metal cools below its ignition temperature. This type of extinguisher was invented in the late 1940s as a cartridge-operated unit, although stored pressure versions are now popular. Common sizes are portable and wheeled.
Cleanser
Since at leastmedieval
In the history of Europe, the Middle Ages or medieval period lasted approximately from the late 5th to the late 15th centuries, similar to the Post-classical, post-classical period of World history (field), global history. It began with t ...
times, people have used salt as a cleansing agent rubbed on household surfaces. It is also used in many brands of shampoo
Shampoo () is a hair care product, typically in the form of a viscous liquid, that is used for cleaning hair. Less commonly, shampoo is available in solid bar format. Shampoo is used by applying it to wet hair, massaging the product into the ...
, toothpaste and popularly to de-ice driveways and patches of ice.
Optical usage
Defect-free NaCl crystals have an optical transmittance of about 90% for infrared light, specifically between 200 nm and 20 µm. They were therefore used in optical components (windows and prisms) operating in that spectral range, where few non-absorbing alternatives exist and where requirements for absence of microscopic inhomogeneities are less strict than in the visible range. While inexpensive, NaCl crystals are soft andhygroscopic
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substance ...
– when exposed to the ambient air, they gradually cover with "frost". This limits application of NaCl to dry environments, vacuum sealed assembly areas or for short-term uses such as prototyping. Nowadays materials like zinc selenide
Zinc selenide (ZnSe) is a light-yellow, solid compound comprising zinc (Zn) and selenium (Se). It is an intrinsic semiconductor with a band gap of about 2.70 eV at . ZnSe rarely occurs in nature, and is found in the mineral that was named af ...
(ZnSe), which are stronger mechanically and are less sensitive to moisture, are used instead of NaCl for the infrared spectral range.
Chemistry
Solid sodium chloride

octahedron
In geometry, an octahedron (plural: octahedra, octahedrons) is a polyhedron with eight faces. The term is most commonly used to refer to the regular octahedron, a Platonic solid composed of eight equilateral triangles, four of which meet at ea ...
. In the language of close-packing
In geometry, close-packing of equal spheres is a dense arrangement of congruent spheres in an infinite, regular arrangement (or lattice). Carl Friedrich Gauss proved that the highest average density – that is, the greatest fraction of space occu ...
, the larger chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride salts ...
ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
s (167 pm in size) are arranged in a cubic array whereas the smaller sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable iso ...
ions (116 pm) fill all the cubic gaps (octahedral voids) between them. This same basic structure is found in many other compounds and is commonly known as the halite
Halite (), commonly known as rock salt, is a type of salt, the mineral (natural) form of sodium chloride ( Na Cl). Halite forms isometric crystals. The mineral is typically colorless or white, but may also be light blue, dark blue, purple, p ...
or rock-salt crystal structure. It can be represented as a face-centered cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties of ...
(fcc) lattice with a two-atom basis or as two interpenetrating face centered cubic lattices. The first atom is located at each lattice point, and the second atom is located halfway between lattice points along the fcc unit cell edge.
Solid sodium chloride has a melting point of 801 °C. Thermal conductivity
The thermal conductivity of a material is a measure of its ability to conduct heat. It is commonly denoted by k, \lambda, or \kappa.
Heat transfer occurs at a lower rate in materials of low thermal conductivity than in materials of high thermal ...
of sodium chloride as a function of temperature has a maximum of 2.03 W/(cm K) at and decreases to 0.069 at . It also decreases with doping.
Atomic-resolution real-time video imaging allows visualization of the initial stage of crystal nucleation of sodium chloride.
Aqueous solutions
The attraction between the Na+ and Cl− ions in the solid is so strong that only highly polar solvents like water dissolve NaCl well. When dissolved in water, the sodium chloride framework disintegrates as the Na+ and Cl− ions become surrounded by polar water molecules. These solutions consist of
When dissolved in water, the sodium chloride framework disintegrates as the Na+ and Cl− ions become surrounded by polar water molecules. These solutions consist of metal aquo complex
In chemistry, metal aquo complexes are coordination compounds containing metal ions with only water as a ligand. These complexes are the predominant species in aqueous solutions of many metal salts, such as metal nitrates, sulfates, and perchlorat ...
with the formula a(H2O)8sup>+, with the Na–O distance of 250 pm. The chloride ions are also strongly solvated, each being surrounded by an average of six molecules of water.Lincoln, S. F.; Richens, D. T. and Sykes, A. G. (2003) "Metal Aqua Ions" Comprehensive Coordination Chemistry II Volume 1, pp. 515–555. . Solutions of sodium chloride have very different properties from pure water. The eutectic point
A eutectic system or eutectic mixture ( ) is a homogeneous mixture that has a melting point lower than those of the constituents. The lowest possible melting point over all of the mixing ratios of the constituents is called the ''eutectic tempe ...
is for 23.31% mass fraction of salt, and the boiling point of saturated salt solution is near .Elvers, B. ''et al.'' (ed.) (1991) ''Ullmann's Encyclopedia of Industrial Chemistry'', 5th ed. Vol. A24, Wiley, p. 319, . From cold solutions, salt crystallises as the dihydrate
In chemistry, a hydrate is a substance that contains water or its constituent elements. The chemical state of the water varies widely between different classes of hydrates, some of which were so labeled before their chemical structure was understo ...
NaCl·2H2O.
pH of sodium chloride solutions
The pH of a sodium chloride solution remains ≈7 due to the extremely weak basicity of the Cl− ion, which is the conjugate base of the strong acid HCl. In other words, NaCl has no effect on system pH in diluted solutions where the effects of ionic strength and activity coefficients are negligible.Stoichiometric and structure variants
Common salt has a 1:1 molar ratio of sodium and chlorine. In 2013, compounds of sodium and chloride of differentstoichiometries
Stoichiometry refers to the relationship between the quantities of reactants and products before, during, and following chemical reactions.
Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equals ...
have been discovered; five new compounds were predicted (e.g., Na3Cl, Na2Cl, Na3Cl2, NaCl3, and NaCl7). The existence of some of them has been experimentally confirmed at high pressures: cubic and orthorhombic NaCl3 and two-dimensional metallic tetragonal Na3Cl. This indicates that compounds violating chemical intuition are possible, in simple systems under nonambient conditions.
In 2020, calculations and experiments showed the existence of a hexagonal NaCl. Exotic hexagonal NaCl thin films on the (110) diamond surface were crystallized in the experiment following a theoretical prediction based on ab initio evolutionary algorithm USPEX.
Occurrence
Most of the world's salt is dissolved in theocean
The ocean (also the sea or the world ocean) is the body of salt water that covers approximately 70.8% of the surface of Earth and contains 97% of Earth's water. An ocean can also refer to any of the large bodies of water into which the wo ...
. A lesser amount is found in the Earth's crust
Earth's crust is Earth's thin outer shell of rock, referring to less than 1% of Earth's radius and volume. It is the top component of the lithosphere, a division of Earth's layers that includes the crust and the upper part of the mantle. The ...
as the water-soluble mineral halite
Halite (), commonly known as rock salt, is a type of salt, the mineral (natural) form of sodium chloride ( Na Cl). Halite forms isometric crystals. The mineral is typically colorless or white, but may also be light blue, dark blue, purple, p ...
(rock salt), and a tiny amount exists as suspended sea salt
Sea salt is salt that is produced by the evaporation of seawater. It is used as a seasoning in foods, cooking, cosmetics and for preserving food. It is also called bay salt, solar salt, or simply salt. Like mined rock salt, production of sea sa ...
particles in the atmosphere. These particles are the dominant cloud condensation nuclei far out at sea, which allow the formation of cloud
In meteorology, a cloud is an aerosol consisting of a visible mass of miniature liquid droplets, frozen crystals, or other particles suspended in the atmosphere of a planetary body or similar space. Water or various other chemicals may co ...
s in otherwise non-polluted air
The atmosphere of Earth is the layer of gases, known collectively as air, retained by Earth's gravity that surrounds the planet and forms its planetary atmosphere. The atmosphere of Earth protects life on Earth by creating pressure allowing f ...
.
Production
Salt is currentlymass-produced
Mass production, also known as flow production or continuous production, is the production of substantial amounts of standardized products in a constant flow, including and especially on assembly lines. Together with job production and ba ...
by evaporation
Evaporation is a type of vaporization that occurs on the surface of a liquid as it changes into the gas phase. High concentration of the evaporating substance in the surrounding gas significantly slows down evaporation, such as when humidi ...
of seawater
Seawater, or salt water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has appro ...
or brine
Brine is a high-concentration solution of salt (NaCl) in water (H2O). In diverse contexts, ''brine'' may refer to the salt solutions ranging from about 3.5% (a typical concentration of seawater, on the lower end of that of solutions used for br ...
from brine well
A salt well (or brine well) is used to mine salt from caverns or deposits. Water is used as a solution to dissolve the salt or halite deposits so that they can be extracted by pipe to an evaporation process, which results in a brine or dry produc ...
s and salt lakes
A salt lake or saline lake is a landlocked body of water that has a concentration of salt (chemistry), salts (typically sodium chloride) and other dissolved minerals significantly higher than most lakes (often defined as at least three grams of ...
. Mining
Mining is the extraction of valuable minerals or other geological materials from the Earth, usually from an ore body, lode, vein, seam, reef, or placer deposit. The exploitation of these deposits for raw material is based on the economic via ...
of rock salt is also a major source. China is the world's main supplier of salt.Kostick, Dennis S. (October 2010"Salt"
in ''U.S. Geological Survey, 2008 Minerals Yearbook'' In 2017, world production was estimated at 280 million
tonne
The tonne ( or ; symbol: t) is a unit of mass equal to 1000 kilograms. It is a non-SI unit accepted for use with SI. It is also referred to as a metric ton to distinguish it from the non-metric units of the short ton ( United State ...
s, the top five producers (in million tonnes) being China (68.0), United States (43.0), India (26.0), Germany (13.0), and Canada (13.0).U.S. Geological Survey
potassium
Potassium is the chemical element with the symbol K (from Neo-Latin ''kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmosphe ...
mining.
United States
The United States of America (U.S.A. or USA), commonly known as the United States (U.S. or US) or America, is a country primarily located in North America. It consists of 50 states, a federal district, five major unincorporated territorie ...
File:Dead-Sea---Salt-Evaporation-Ponds.jpg, Jordan
Jordan ( ar, الأردن; tr. ' ), officially the Hashemite Kingdom of Jordan,; tr. ' is a country in Western Asia. It is situated at the crossroads of Asia, Africa, and Europe, within the Levant region, on the East Bank of the Jordan Rive ...
ian and Israel
Israel (; he, יִשְׂרָאֵל, ; ar, إِسْرَائِيل, ), officially the State of Israel ( he, מְדִינַת יִשְׂרָאֵל, label=none, translit=Medīnat Yīsrāʾēl; ), is a country in Western Asia. It is situated ...
i salt evaporation ponds at the south end of the Dead Sea
The Dead Sea ( he, יַם הַמֶּלַח, ''Yam hamMelaḥ''; ar, اَلْبَحْرُ الْمَيْتُ, ''Āl-Baḥrū l-Maytū''), also known by other names, is a salt lake bordered by Jordan to the east and Israel and the West Bank ...
.
File:Piles of Salt Salar de Uyuni Bolivia Luca Galuzzi 2006 a.jpg, Mounds of salt, Salar de Uyuni, Bolivia
, image_flag = Bandera de Bolivia (Estado).svg
, flag_alt = Horizontal tricolor (red, yellow, and green from top to bottom) with the coat of arms of Bolivia in the center
, flag_alt2 = 7 × 7 square p ...
.
See also
* Biosalinity * Edible salt (table salt) *Halite
Halite (), commonly known as rock salt, is a type of salt, the mineral (natural) form of sodium chloride ( Na Cl). Halite forms isometric crystals. The mineral is typically colorless or white, but may also be light blue, dark blue, purple, p ...
, the mineral form of sodium chloride
* Health effects of salt
The health effects of salt are the conditions associated with the consumption of either too much or too little salt. Salt is a mineral composed primarily of sodium chloride (NaCl) and is used in food for both preservation and flavor. Sodium ions ...
* Salinity
Salinity () is the saltiness or amount of salt dissolved in a body of water, called saline water (see also soil salinity). It is usually measured in g/L or g/kg (grams of salt per liter/kilogram of water; the latter is dimensionless and equal ...
* Salting the earth
Salting the earth, or sowing with salt, is the ritual of spreading salt on the site of cities razed by conquerers. It originated as a curse on re-inhabitation in the ancient Near East and became a well-established folkloric motif in the Middle ...
* Salt poisoning
Salt poisoning is an intoxication resulting from the excessive intake of sodium (usually as sodium chloride) in either solid form or in solution ( saline water, including brine, brackish water, eating salt, or seawater). Salt poisoning suffici ...
References
*Cited sources
*External links
Salt
United States Geological Survey
The United States Geological Survey (USGS), formerly simply known as the Geological Survey, is a scientific agency of the United States government. The scientists of the USGS study the landscape of the United States, its natural resources, ...
Statistics and Information
*
* Calculatorssurface tensions
an
densities, molarities and molalities
of aqueous NaCl (and other salts)
{{DEFAULTSORT:Sodium Chloride Alkali metal chlorides Chlorides Deliquescent substances Household chemicals Metal halides Sodium compounds Sodium minerals Rock salt crystal structure Ophthalmology drugs