N-linked Glycosylation on:
[Wikipedia]
[Google]
[Amazon]
 ''N''-linked glycosylation, is the attachment of an
''N''-linked glycosylation, is the attachment of an
 The biosynthesis of ''N''-linked glycans occurs via 3 major steps:
#Synthesis of dolichol-linked precursor oligosaccharide
#En bloc transfer of precursor oligosaccharide to protein
#Processing of the oligosaccharide
Synthesis, en bloc transfer and initial trimming of precursor
The biosynthesis of ''N''-linked glycans occurs via 3 major steps:
#Synthesis of dolichol-linked precursor oligosaccharide
#En bloc transfer of precursor oligosaccharide to protein
#Processing of the oligosaccharide
Synthesis, en bloc transfer and initial trimming of precursor
 ''N''-glycan processing is carried out in endoplasmic reticulum and the Golgi body. Initial trimming of the precursor molecule occurs in the ER and the subsequent processing occurs in the Golgi.
Upon transferring the completed glycan onto the nascent polypeptide, two glucose residues are removed from the structure. Enzymes known as glycosidases remove some sugar residues. These enzymes can break glycosidic linkages by using a water molecule. These enzymes are exoglycosidases as they only work on
''N''-glycan processing is carried out in endoplasmic reticulum and the Golgi body. Initial trimming of the precursor molecule occurs in the ER and the subsequent processing occurs in the Golgi.
Upon transferring the completed glycan onto the nascent polypeptide, two glucose residues are removed from the structure. Enzymes known as glycosidases remove some sugar residues. These enzymes can break glycosidic linkages by using a water molecule. These enzymes are exoglycosidases as they only work on  *High-mannose is, in essence, just two ''N''-acetylglucosamines with many mannose residues, often almost as many as are seen in the precursor oligosaccharides before it is attached to the protein.
*Complex oligosaccharides are so named because they can contain almost any number of the other types of saccharides, including more than the original two ''N''-acetylglucosamines.
*Hybrid oligosaccharides contain a mannose residues on one side of the branch, while on the other side a ''N''-acetylglucosamine initiates a complex branch.
The order of addition of sugars to the growing glycan chains is determined by the substrate specificities of the enzymes and their access to the substrate as they move through
*High-mannose is, in essence, just two ''N''-acetylglucosamines with many mannose residues, often almost as many as are seen in the precursor oligosaccharides before it is attached to the protein.
*Complex oligosaccharides are so named because they can contain almost any number of the other types of saccharides, including more than the original two ''N''-acetylglucosamines.
*Hybrid oligosaccharides contain a mannose residues on one side of the branch, while on the other side a ''N''-acetylglucosamine initiates a complex branch.
The order of addition of sugars to the growing glycan chains is determined by the substrate specificities of the enzymes and their access to the substrate as they move through
 The importance of ''N''-linked glycosylation is becoming increasingly evident in the field of
The importance of ''N''-linked glycosylation is becoming increasingly evident in the field of
GlycoEP
In silico Platform for Prediction of ''N''-, ''O''- and ''C''-Glycosites in Eukaryotic Protein Sequences * {{cite journal , vauthors = Maverakis E, Kim K, Shimoda M, Gershwin ME, Patel F, Wilken R, Raychaudhuri S, Ruhaak LR, Lebrilla CB , title = Glycans in the immune system and The Altered Glycan Theory of Autoimmunity: a critical review , journal = Journal of Autoimmunity , volume = 57 , pages = 1–13 , date = February 2015 , pmid = 25578468 , pmc = 4340844 , doi = 10.1016/j.jaut.2014.12.002 Organic chemistry Biochemistry
oligosaccharide
An oligosaccharide (/ˌɑlɪgoʊˈsækəˌɹaɪd/; from the Greek ὀλίγος ''olígos'', "a few", and σάκχαρ ''sácchar'', "sugar") is a saccharide polymer containing a small number (typically two to ten) of monosaccharides (simple sug ...
, a carbohydrate consisting of several sugar molecules, sometimes also referred to as glycan
The terms glycans and polysaccharides are defined by IUPAC as synonyms meaning "compounds consisting of a large number of monosaccharides linked glycosidically". However, in practice the term glycan may also be used to refer to the carbohydrate ...
, to a nitrogen atom (the amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it i ...
nitrogen of an asparagine
Asparagine (symbol Asn or N) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form under biological conditions), an α-carboxylic acid group (which is in the depro ...
(Asn) residue of a protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
), in a process called ''N''-glycosylation, studied in biochemistry
Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology and ...
. This type of linkage is important for both the structure and function of many eukaryotic proteins. The ''N''-linked glycosylation
Glycosylation is the reaction in which a carbohydrate (or ' glycan'), i.e. a glycosyl donor, is attached to a hydroxyl or other functional group of another molecule (a glycosyl acceptor) in order to form a glycoconjugate. In biology (but not al ...
process occurs in eukaryotes
Eukaryotes () are organisms whose cells have a nucleus. All animals, plants, fungi, and many unicellular organisms, are Eukaryotes. They belong to the group of organisms Eukaryota or Eukarya, which is one of the three domains of life. Bacter ...
and widely in archaea
Archaea ( ; singular archaeon ) is a domain of single-celled organisms. These microorganisms lack cell nuclei and are therefore prokaryotes. Archaea were initially classified as bacteria, receiving the name archaebacteria (in the Archaeba ...
, but very rarely in bacteria
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one biological cell. They constitute a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria were am ...
. The nature of ''N''-linked glycans attached to a glycoprotein is determined by the protein and the cell in which it is expressed. It also varies across species
In biology, a species is the basic unit of classification and a taxonomic rank of an organism, as well as a unit of biodiversity. A species is often defined as the largest group of organisms in which any two individuals of the appropriat ...
. Different species synthesize different types of ''N''-linked glycan.
Energetics of bond formation
There are two types of bonds involved in a glycoprotein: bonds between the saccharides residues in the glycan and the linkage between the glycan chain and the protein molecule. The sugar moieties are linked to one another in the glycan chain viaglycosidic bonds
A glycosidic bond or glycosidic linkage is a type of covalent bond that joins a carbohydrate (sugar) molecule to another group, which may or may not be another carbohydrate.
A glycosidic bond is formed between the hemiacetal or hemiketal gr ...
. These bonds are typically formed between carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon ma ...
s 1 and 4 of the sugar molecules. The formation of glycosidic bond is energetically unfavourable, therefore the reaction is coupled to the hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysi ...
of two ATP molecules.
On the other hand, the attachment of a glycan residue to a protein requires the recognition of a consensus sequence
In molecular biology and bioinformatics, the consensus sequence (or canonical sequence) is the calculated order of most frequent residues, either nucleotide or amino acid, found at each position in a sequence alignment. It serves as a simplified r ...
. ''N''-linked glycans are almost always attached to the nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
atom of an asparagine (Asn) side chain that is present as a part of Asn–X– Ser/ Thr consensus sequence, where X is any amino acid except proline
Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group but is rather a secondary amine. The secondary amine nitrogen is in the p ...
(Pro).
In animal cells, the glycan attached to the asparagine is almost inevitably ''N''-acetylglucosamine (GlcNAc) in the β-configuration. This β-linkage is similar to glycosidic bond between the sugar moieties in the glycan structure as described above. Instead of being attached to a sugar hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydrox ...
group, the anomeric carbon
In carbohydrate chemistry, a pair of anomers () is a pair of near-identical stereoisomers that differ at only the anomeric carbon, the carbon that bears the aldehyde or ketone functional group in the sugar's open-chain form. However, in order fo ...
atom is attached to an amide nitrogen. The energy required for this linkage comes from the hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysi ...
of a pyrophosphate molecule.
Biosynthesis
oligosaccharide
An oligosaccharide (/ˌɑlɪgoʊˈsækəˌɹaɪd/; from the Greek ὀλίγος ''olígos'', "a few", and σάκχαρ ''sácchar'', "sugar") is a saccharide polymer containing a small number (typically two to ten) of monosaccharides (simple sug ...
occurs in the endoplasmic reticulum
The endoplasmic reticulum (ER) is, in essence, the transportation system of the eukaryotic cell, and has many other important functions such as protein folding. It is a type of organelle made up of two subunits – rough endoplasmic reticulum ...
(ER). Subsequent processing and modification of the oligosaccharide chain are carried out in the Golgi apparatus
The Golgi apparatus (), also known as the Golgi complex, Golgi body, or simply the Golgi, is an organelle found in most eukaryotic cells. Part of the endomembrane system in the cytoplasm, it packages proteins into membrane-bound vesicles i ...
.
The synthesis of glycoproteins is thus spatially separated in different cellular compartments. Therefore, the type of ''N''-glycan synthesised, depends on its accessibility to the different enzymes present within these cellular compartments.
However, in spite of the diversity, all ''N''-glycans are synthesized through a common pathwamannose
Mannose is a sugar monomer of the aldohexose series of carbohydrates. It is a C-2 epimer of glucose. Mannose is important in human metabolism, especially in the glycosylation of certain proteins. Several congenital disorders of glycosylat ...
residues. This core glycan is then elaborated and modified further, resulting in a diverse range of ''N''-glycan structures.
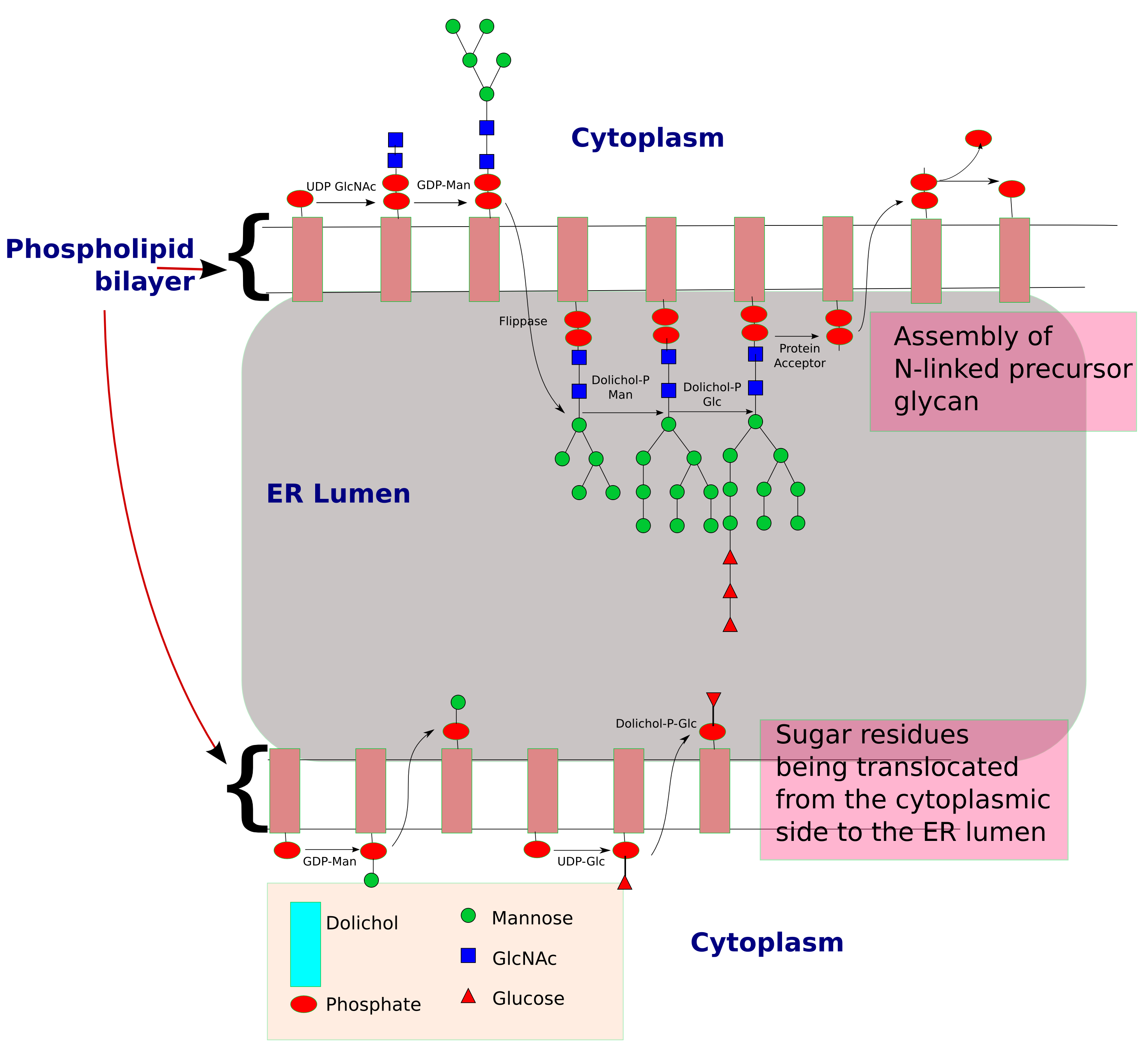
Synthesis of precursor oligosaccharide
The process of ''N''-linked glycosylation starts with the formation of dolichol-linked GlcNAc sugar. Dolichol is alipid
Lipids are a broad group of naturally-occurring molecules which includes fats, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E and K), monoglycerides, diglycerides, phospholipids, and others. The functions of lipids in ...
molecule composed of repeating isoprene
Isoprene, or 2-methyl-1,3-butadiene, is a common volatile organic compound with the formula CH2=C(CH3)−CH=CH2. In its pure form it is a colorless volatile liquid. Isoprene is an unsaturated hydrocarbon. It is produced by many plants and animals ...
units. This molecule is found attached to the membrane of the ER. Sugar molecules are attached to the dolichol through a pyrophosphate linkage (one phosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid .
The phosphate or orthophosphate ion is derived from phosph ...
was originally linked to dolichol, and the second phosphate came from the nucleotide
Nucleotides are organic molecules consisting of a nucleoside and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecu ...
sugar). The oligosaccharide chain is then extended through the addition of various sugar molecules in a stepwise manner to form a precursor oligosaccharide.
The assembly of this precursor oligosaccharide occurs in two phases: Phase I and II. Phase I takes place on the cytoplasmic
In cell biology, the cytoplasm is all of the material within a eukaryotic cell, enclosed by the cell membrane, except for the cell nucleus. The material inside the nucleus and contained within the nuclear membrane is termed the nucleoplasm. The ...
side of the ER and Phase II takes place on the luminal side of the ER.
The precursor molecule, ready to be transferred to a protein, consists of 2 GlcNAc, 9 mannose, and 3 glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, u ...
molecules.

Transfer of glycan to protein
Once the precursor oligosaccharide is formed, the completed glycan is then transferred to the nascentpolypeptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.
...
in the lumen of the ER membrane. This reaction is driven by the energy released from the cleavage of the pyrophosphate bond between the dolichol-glycan molecule.
There are three conditions to fulfill before a glycan is transferred to a nascent polypeptide:
*Asparagine must be located in a specific consensus sequence in the primary structure
Protein primary structure is the linear sequence of amino acids in a peptide or protein. By convention, the primary structure of a protein is reported starting from the amino-terminal (N) end to the carboxyl-terminal (C) end. Protein biosynth ...
(Asn–X–Ser or Asn–X–Thr or in rare instances Asn–X–Cys).
*Asparagine must be located appropriately in the three-dimensional structure of the protein (Sugars are polar molecules
In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively charged end.
Polar molecules must contain one or more polar ...
and thus need to be attached to asparagine located on the surface of the protein and not buried within the protein)
*Asparagine must be found in the luminal side of the endoplasmic reticulum for ''N''-linked glycosylation to be initiated. Target residues are either found in secretory proteins or in the regions of transmembrane protein
A transmembrane protein (TP) is a type of integral membrane protein that spans the entirety of the cell membrane. Many transmembrane proteins function as gateways to permit the transport of specific substances across the membrane. They frequent ...
that face the lumen.
Oligosaccharyltransferase is the enzyme responsible for the recognition of the consensus sequence and the transfer of the precursor glycan to a polypeptide acceptor which is being translated in the endoplasmic reticulum lumen. ''N''-linked glycosylation is, therefore, a co-translational event
Processing of glycan
 ''N''-glycan processing is carried out in endoplasmic reticulum and the Golgi body. Initial trimming of the precursor molecule occurs in the ER and the subsequent processing occurs in the Golgi.
Upon transferring the completed glycan onto the nascent polypeptide, two glucose residues are removed from the structure. Enzymes known as glycosidases remove some sugar residues. These enzymes can break glycosidic linkages by using a water molecule. These enzymes are exoglycosidases as they only work on
''N''-glycan processing is carried out in endoplasmic reticulum and the Golgi body. Initial trimming of the precursor molecule occurs in the ER and the subsequent processing occurs in the Golgi.
Upon transferring the completed glycan onto the nascent polypeptide, two glucose residues are removed from the structure. Enzymes known as glycosidases remove some sugar residues. These enzymes can break glycosidic linkages by using a water molecule. These enzymes are exoglycosidases as they only work on monosaccharide
Monosaccharides (from Greek '' monos'': single, '' sacchar'': sugar), also called simple sugars, are the simplest forms of sugar and the most basic units (monomers) from which all carbohydrates are built.
They are usually colorless, water- so ...
residues located at the non-reducing end of the glycan. This initial trimming step is thought to act as a quality control step in the ER to monitor protein folding
Protein folding is the physical process by which a protein chain is translated to its native three-dimensional structure, typically a "folded" conformation by which the protein becomes biologically functional. Via an expeditious and reproduc ...
.
Once the protein is folded correctly, two glucose residues are removed by glucosidase I and II. The removal of the final third glucose residue signals that the glycoprotein is ready for transit from the ER to the ''cis''-Golgi. ER mannosidase catalyses the removal of this final glucose. However, if the protein is not folded properly, the glucose residues are not removed and thus the glycoprotein can't leave the endoplasmic reticulum. A chaperone protein (calnexin
Calnexin (CNX) is 67kDaintegral protein (that appears variously as a 90kDa, 80kDa, or 75kDa band on western blotting depending on the source of the antibody) of the endoplasmic reticulum (ER). It consists of a large (50 kDa) N-terminal calcium- ...
/calreticulin
Calreticulin also known as calregulin, CRP55, CaBP3, calsequestrin-like protein, and endoplasmic reticulum resident protein 60 (ERp60) is a protein that in humans is encoded by the ''CALR'' gene.
Calreticulin is a multifunctional soluble prote ...
) binds to the unfolded or partially folded protein to assist protein folding.
The next step involves further addition and removal of sugar residues in the cis-Golgi. These modifications are catalyzed by glycosyltransferases and glycosidases respectively. In the ''cis''-Golgi, a series of mannosidases remove some or all of the four mannose residues in α-1,2 linkages. Whereas in the medial portion of the Golgi, glycosyltransferases add sugar residues to the core glycan structure, giving rise to the three main types of glycans: high mannose, hybrid and complex glycans.
secretory pathway 440px
Secretion is the movement of material from one point to another, such as a secreted chemical substance from a cell or gland. In contrast, excretion is the removal of certain substances or waste products from a cell or organism. The classica ...
. Thus, the organization of this machinery within a cell plays an important role in determining which glycans are made.
Enzymes in the Golgi
Golgi enzymes play a key role in determining the synthesis of the various types of glycans. The order of action of the enzymes is reflected in their position in the Golgi stack:In archaea and prokaryotes
Similar ''N''-glycan biosynthesis pathway have been found in prokaryotes and Archaea. However, compared to eukaryotes, the final glycan structure in eubacteria and archaea does not seem to differ much from the initial precursor made in the endoplasmic reticulum. In eukaryotes, the original precursor oligosaccharide is extensively modified en route to the cell surface.Function
''N''-linked glycans have intrinsic and extrinsic functions. Within the immune system, the ''N''-linked glycans on an immune cell's surface will help dictate that migration pattern of the cell, e.g. immune cells that migrate to the skin have specific glycosylations that favor homing to that site. The glycosylation patterns on the various immunoglobulins including IgE, IgM, IgD, IgA, and IgG bestow them with unique effector functions by altering their affinities for Fc and other immune receptors. Glycans may also be involved in "self" and "non self" discrimination, which may be relevant to the pathophysiology of various autoimmune diseases. In some cases, interaction between the N-glycan and the protein stabilizes the protein through complex electronic effects.Clinical significance
Changes in ''N''-linked glycosylation has been associated with different diseases includingrheumatoid arthritis
Rheumatoid arthritis (RA) is a long-term autoimmune disorder that primarily affects joints. It typically results in warm, swollen, and painful joints. Pain and stiffness often worsen following rest. Most commonly, the wrist and hands are inv ...
, type 1 diabetes
Type 1 diabetes (T1D), formerly known as juvenile diabetes, is an autoimmune disease that originates when cells that make insulin (beta cells) are destroyed by the immune system. Insulin is a hormone required for the cells to use blood sugar f ...
, Crohn's disease
Crohn's disease is a type of inflammatory bowel disease (IBD) that may affect any segment of the gastrointestinal tract. Symptoms often include abdominal pain, diarrhea (which may be bloody if inflammation is severe), fever, abdominal distensi ...
, and cancers.
Mutations in eighteen genes involved in ''N''-linked glycosylation result in a variety of diseases, most of which involve the nervous system
In biology, the nervous system is the highly complex part of an animal that coordinates its actions and sensory information by transmitting signals to and from different parts of its body. The nervous system detects environmental changes ...
.
Importance in therapeutic proteins
Many therapeutic proteins in the market areantibodies
An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein used by the immune system to identify and neutralize foreign objects such as pathogenic bacteria and viruses. The antibody recognizes a unique molecule of ...
, which are ''N''-linked glycoproteins. For example, Etanercept
Etanercept, sold under the brand name Enbrel among others, is a biologic medical product that is used to treat autoimmune diseases by interfering with tumor necrosis factor (TNF), a soluble inflammatory cytokine, by acting as a TNF inhibitor. It ...
, Infliximab
Infliximab, a chimeric monoclonal antibody, sold under the brand name Remicade among others, is a medication used to treat a number of autoimmune diseases. This includes Crohn's disease, ulcerative colitis, rheumatoid arthritis, ankylosing spon ...
and Rituximab are ''N''-glycosylated therapeutic proteins.
pharmaceuticals
A medication (also called medicament, medicine, pharmaceutical drug, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy ( pharmacotherapy) is an important part of the medical field and re ...
. Although bacterial or yeast protein production
Protein production is the biotechnological process of generating a specific protein. It is typically achieved by the manipulation of gene expression in an organism such that it expresses large amounts of a recombinant gene. This includes the t ...
systems have significant potential advantages such as high yield and low cost, problems arise when the protein of interest is a glycoprotein. Most prokaryotic expression systems such as '' E. coli'' cannot carry out post-translational modifications
Post-translational modification (PTM) is the covalent and generally enzymatic modification of proteins following protein biosynthesis. This process occurs in the endoplasmic reticulum and the golgi apparatus. Proteins are synthesized by ribo ...
. On the other hand, eukaryotic expression hosts such as yeast and animal cells, have different glycosylation patterns. The proteins produced in these expression hosts are often not identical to human protein and thus, cause immunogenic reactions in patients. For example, '' S.cerevisiae'' (yeast) often produce high-mannose glycans which are immunogenic.
Non-human mammalian expression systems such as CHO or NS0 cells have the machinery required to add complex, human-type glycans. However, glycans produced in these systems can differ from glycans produced in humans, as they can be capped with both ''N''-glycolylneuraminic acid (Neu5Gc) and ''N''-acetylneuraminic acid (Neu5Ac), whereas human cells only produce glycoproteins containing ''N''-acetylneuraminic acid. Furthermore, animal cells can also produce glycoproteins containing the galactose-alpha-1,3-galactose epitope, which can induce serious allergenic reactions, including anaphylactic shock
Anaphylaxis is a serious, potentially fatal allergic reaction and medical emergency that is rapid in onset and requires immediate medical attention regardless of use of emergency medication on site. It typically causes more than one of the follow ...
, in people who have Alpha-gal allergy
Alpha-gal allergy — or mammalian meat allergy (MMA) — is a type of meat allergy characterized by a delayed onset of symptoms (3–8 hours) after ingesting mammalian meat and resulting from past exposure to tick bites. It was first reported in ...
.
These drawbacks have been addressed by several approaches such as eliminating the pathways that produce these glycan structures through genetic knockouts. Furthermore, other expression systems have been genetically engineered to produce therapeutic glycoproteins with human-like ''N''-linked glycans. These include yeasts such as '' Pichia pastoris'', insect cell lines, green plants, and even bacteria.
See also
*Glycosylation
Glycosylation is the reaction in which a carbohydrate (or ' glycan'), i.e. a glycosyl donor, is attached to a hydroxyl or other functional group of another molecule (a glycosyl acceptor) in order to form a glycoconjugate. In biology (but not al ...
* ''O''-linked glycosylation
*Gene expression
Gene expression is the process by which information from a gene is used in the synthesis of a functional gene product that enables it to produce end products, protein or non-coding RNA, and ultimately affect a phenotype, as the final effect. T ...
* ''N''-Glycosyltransferase
References
External links
GlycoEP
In silico Platform for Prediction of ''N''-, ''O''- and ''C''-Glycosites in Eukaryotic Protein Sequences * {{cite journal , vauthors = Maverakis E, Kim K, Shimoda M, Gershwin ME, Patel F, Wilken R, Raychaudhuri S, Ruhaak LR, Lebrilla CB , title = Glycans in the immune system and The Altered Glycan Theory of Autoimmunity: a critical review , journal = Journal of Autoimmunity , volume = 57 , pages = 1–13 , date = February 2015 , pmid = 25578468 , pmc = 4340844 , doi = 10.1016/j.jaut.2014.12.002 Organic chemistry Biochemistry