Mucoadhesion on:
[Wikipedia]
[Google]
[Amazon]
Mucoadhesion describes the attractive forces between a biological material and
 Fracture theory:
Fracture theory:
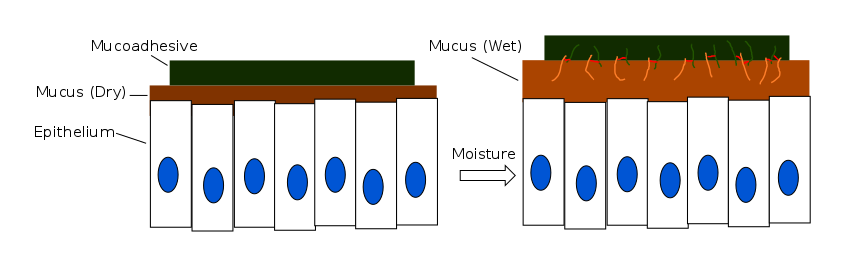 The consolidation stage of mucoadhesion involves the establishment of adhesive interactions to reinforce strong or prolonged adhesion. When moisture is present, mucoadhesive materials become activated and the system becomes plasticized. This stimulus allows the mucoadhesive molecules to separate and break free while proceeding to link up by weak van der Waals and
The consolidation stage of mucoadhesion involves the establishment of adhesive interactions to reinforce strong or prolonged adhesion. When moisture is present, mucoadhesive materials become activated and the system becomes plasticized. This stimulus allows the mucoadhesive molecules to separate and break free while proceeding to link up by weak van der Waals and
 The Macromolecular Interpenetration theory, also known as the diffusion theory, states that the mucoadhesive molecules and mucus glycoproteins mutually interact by means of interpenetration of their chains and the forming of secondary semi-permanent adhesive bonds. It is necessary that the mucoadhesive device has features or properties that favor both chemical and mechanical interactions for the macromolecular interpenetration theory to take place. Molecules that can present mucoadhesive properties are molecules with hydrogen bond building groups, high molecular weight, flexible chains, and surface active properties.
It is perceived that increase in adhesion force is associated with the degree of penetration of polymer chains. Literature states that the degree of penetration required for efficient bioadhesive bonds lies in the range of 0.2-0.5μm. The following equation can be used to estimate the degree of penetration of polymer and mucus chains:
with as contact time and as the
The Macromolecular Interpenetration theory, also known as the diffusion theory, states that the mucoadhesive molecules and mucus glycoproteins mutually interact by means of interpenetration of their chains and the forming of secondary semi-permanent adhesive bonds. It is necessary that the mucoadhesive device has features or properties that favor both chemical and mechanical interactions for the macromolecular interpenetration theory to take place. Molecules that can present mucoadhesive properties are molecules with hydrogen bond building groups, high molecular weight, flexible chains, and surface active properties.
It is perceived that increase in adhesion force is associated with the degree of penetration of polymer chains. Literature states that the degree of penetration required for efficient bioadhesive bonds lies in the range of 0.2-0.5μm. The following equation can be used to estimate the degree of penetration of polymer and mucus chains:
with as contact time and as the


mucus
Mucus ( ) is a slippery aqueous secretion produced by, and covering, mucous membranes. It is typically produced from cells found in mucous glands, although it may also originate from mixed glands, which contain both serous and mucous cells. It is ...
or mucous membrane
A mucous membrane or mucosa is a membrane that lines various cavities in the body of an organism and covers the surface of internal organs. It consists of one or more layers of epithelial cells overlying a layer of loose connective tissue. It is ...
. Mucous membranes adhere to epithelial surfaces such as the gastrointestinal tract (GI-tract), the vagina, the lung, the eye, etc. They are generally hydrophilic
A hydrophile is a molecule or other molecular entity that is attracted to water molecules and tends to be dissolved by water.Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon'' Oxford: Clarendon Press.
In contrast, hydrophobes are no ...
as they contain many hydrogen macromolecules due to the large amount of water (approximately 95%) within its composition. However, mucin also contains glycoproteins
Glycoproteins are proteins which contain oligosaccharide chains covalently attached to amino acid side-chains. The carbohydrate is attached to the protein in a cotranslational or posttranslational modification. This process is known as glycos ...
that enable the formation of a gel-like substance. Understanding the hydrophilic bonding and adhesion mechanisms of mucus to biological material is of utmost importance in order to produce the most efficient applications. For example, in drug delivery
Drug delivery refers to approaches, formulations, manufacturing techniques, storage systems, and technologies involved in transporting a pharmaceutical compound to its target site to achieve a desired therapeutic effect. Principles related to d ...
systems, the mucus layer must be penetrated in order to effectively transport micro- or nanosized drug particles into the body. Bioadhesion is the mechanism by which two biological materials are held together by interfacial forces.
Mucoadhesive bondings
Mucoadhesion involves several types of bonding mechanisms, and it is the interaction between each process that allows for the adhesive process. The major categories are wetting theory, adsorption theory, diffusion theory, electrostatic theory, and fracture theory. Specific processes include mechanical interlocking, electrostatic, diffusion interpenetration, adsorption and fracture processes.Bonding mechanisms
Wetting theory:Wetting
Wetting is the ability of a liquid to maintain contact with a solid surface, resulting from intermolecular interactions when the two are brought together. This happens in presence of a gaseous phase or another liquid phase not miscible with th ...
is the oldest and most prevalent theory of adhesion. The adhesive components in a liquid solution anchor themselves in irregularities on the substrate and eventually harden, providing sites on which to adhere. Surface tension effects restrict the movement of the adhesive along the surface of the substrate, and is related to the thermodynamic work of adhesion by Dupre's Equation. Measuring the affinity of the adhesive for the substrate is performed by determining the contact angle. Contact angles closer to zero indicate a more wettable interaction, and those interactions have a greater spreadability.
Adsorption theory: Adsorption
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a f ...
is another widely accepted theory, where adhesion between the substrate and adhesive is due to primary and secondary bonding. The primary bonds are due to chemisorption, and result in comparatively long lasting covalent and non-covalent bonds. Among covalent bonds disulfide bonds are likely most important. Thiolated polymers – designated thiomers – are mucoadhesive polymers that can form disulfide bonds with cysteine-rich subdomains of mucus glycoproteins. Recently several new classes of polymers have been developed that are capable of forming covalent bonds with mucosal surfaces similarly to thiomers. These polymers have acryloyl, methacryloyl, maleimide, boronate and N‐hydroxy (sulfo) succinimide ester groups in their structure. Among non-covalent bonds likely ionic interactions such as interactions of mucoadhesive chitosans with the anionically charged mucus and Hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
ing are most important. The secondary bonds include weak Van Der Waals forces, and interactions between hydrophobic
In chemistry, hydrophobicity is the physical property of a molecule that is seemingly repelled from a mass of water (known as a hydrophobe). In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, th ...
substructure.
Diffusion theory: The mechanism for diffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical p ...
involves polymer and mucin chains from the adhesive penetrating the matrix of the substrate and forming a semipermanent bond. As the similarities between the adhesive and the substrate increase, so does the degree of mucoadhesion. The bond strength increases with the degree of penetration, increasing the adhesion strength. The penetration rate is determined by the diffusion coefficient
Diffusivity, mass diffusivity or diffusion coefficient is a proportionality constant between the molar flux due to molecular diffusion and the gradient in the concentration of the species (or the driving force for diffusion). Diffusivity is enco ...
, the degree of flexibility of the adsorbate chains, mobility
Mobility may refer to:
Social sciences and humanities
* Economic mobility, ability of individuals or families to improve their economic status
* Geographic mobility, the measure of how populations and goods move over time
* Mobilities, a contemp ...
and contact time. The diffusion mechanism itself is affected by the length of the molecular chains being implanted and cross-linking density, and is driven by a concentration gradient
Molecular diffusion, often simply called diffusion, is the thermal motion of all (liquid or gas) particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size (mass) of ...
.
Electrostatic theory: is an electrostatic
Electrostatics is a branch of physics that studies electric charges at rest (static electricity).
Since classical times, it has been known that some materials, such as amber, attract lightweight particles after rubbing. The Greek word for amber ...
process involving the transfer of electrons across the interface between the substrate and adhesive. The net result is the formation of a double layer of charges that are attracted to each other due to balancing of the Fermi layers, and therefore cause adhesion. This theory only works given the assumption that the substrate and adhesive have different electrostatic surface characteristics.
 Fracture theory:
Fracture theory: Fracture
Fracture is the separation of an object or material into two or more pieces under the action of stress. The fracture of a solid usually occurs due to the development of certain displacement discontinuity surfaces within the solid. If a displa ...
theory is the major mechanism by which to determine the mechanical strength
The field of strength of materials, also called mechanics of materials, typically refers to various methods of calculating the stresses and strains in structural members, such as beams, columns, and shafts. The methods employed to predict the re ...
of a particular mucoadhesive, and describes the force necessary to separate the two materials after mucoadhesion has occurred. Ultimate tensile strength
Ultimate tensile strength (UTS), often shortened to tensile strength (TS), ultimate strength, or F_\text within equations, is the maximum stress that a material can withstand while being stretched or pulled before breaking. In brittle materials t ...
is determined by the separating force and the total surface area of the adhesion, and failure
Failure is the state or condition of not meeting a desirable or intended objective (goal), objective, and may be viewed as the opposite of Success (concept), success. The criteria for failure depends on context, and may be relative to a parti ...
generally occurs in one of the surfaces rather than at the interface. Since the fracture theory only deals with the separation force, the diffusion and penetration of polymers is not accounted for in this mechanism.
Stages of mucoadhesive process
The mucoadhesive process will differ greatly depending on the surface and properties of the adhesive. However, two general steps of the process have been identified: the contact stage and the consolidation stage.Contact stage
The contact stage is the initialwetting
Wetting is the ability of a liquid to maintain contact with a solid surface, resulting from intermolecular interactions when the two are brought together. This happens in presence of a gaseous phase or another liquid phase not miscible with th ...
that occurs between the adhesive and membrane. This can occur mechanically by bringing together the two surfaces, or through the bodily systems, like when particles are deposited in the nasal cavity by inhalation. The principles of initial adsorption
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a f ...
of small molecule adsorbates can be described by DLVO theory
The DLVO theory (named after Boris Derjaguin and Lev Landau, Evert Verwey and Theodoor Overbeek) explains the aggregation of aqueous dispersions quantitatively and describes the force between charged surfaces interacting through a liquid medium.
...
.
Adsorption theory
According toDLVO theory
The DLVO theory (named after Boris Derjaguin and Lev Landau, Evert Verwey and Theodoor Overbeek) explains the aggregation of aqueous dispersions quantitatively and describes the force between charged surfaces interacting through a liquid medium.
...
, particles are held in suspension by a balance of attractive and repulsive forces. This theory can be applied to the adsorption of small molecules like mucoadhesive polymers, on surfaces, like mucus layers. Particles in general experience attractive van der Waals forces
In molecular physics, the van der Waals force is a distance-dependent interaction between atoms or molecules. Unlike ionic bond, ionic or covalent bonds, these attractions do not result from a Chemical bond, chemical electronic bond; they are c ...
that promote coagulation
Coagulation, also known as clotting, is the process by which blood changes from a liquid to a gel, forming a blood clot. It potentially results in hemostasis, the cessation of blood loss from a damaged vessel, followed by repair. The mechanism o ...
; in the context of adsorption
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a f ...
, the particle and mucus layers are naturally attracted. The attractive forces between particles increases with decreasing particle size due to increasing surface-area-to-volume ratio. This increases the strength of van der Waals interactions, so smaller particles should be easier to adsorb onto mucous membranes.
DLVO theory
The DLVO theory (named after Boris Derjaguin and Lev Landau, Evert Verwey and Theodoor Overbeek) explains the aggregation of aqueous dispersions quantitatively and describes the force between charged surfaces interacting through a liquid medium.
...
also explains some of the challenges in establishing contact between particles and mucus layers in mucoadhesion due to their repulsive forces. Surfaces will develop an electrical double layer
A double layer (DL, also called an electrical double layer, EDL) is a structure that appears on the surface of an object when it is exposed to a fluid. The object might be a solid particle, a gas bubble, a liquid droplet, or a porous body. The D ...
if they are in a solution containing ions, as is the case with many bodily systems, creating electrostatic repulsive forces between the adhesive and surface. Steric effects
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions ...
can also hinder particle adsorption to surfaces. Entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynam ...
or disorder of a system will decrease as polymeric mucoadhesives adsorb to surfaces, which makes establishing contact between the adhesive and membrane more difficult. Adhesives with large surface groups will also experience a decrease in entropy as they approach the surface, creating repulsion.
Wettability theory
The initial adsorption of the molecule adhesive will also depend on thewetting
Wetting is the ability of a liquid to maintain contact with a solid surface, resulting from intermolecular interactions when the two are brought together. This happens in presence of a gaseous phase or another liquid phase not miscible with th ...
between the adhesive and membrane. This can be described using Young's equation:
where is the interfacial tension between the membrane and gas or bodily environment, is the interfacial tension between the bioadhesive and membrane, is the interfacial tension between the bioadhesive and bodily environment, and is the contact angle of the bioadhesive on the membrane. The ideal contact angle is 0° meaning the bioadhesive perfectly wets the membrane and good contact is achieved. The interfacial tensions can be measured using common experimental techniques such as a Wilhelmy plate
A Wilhelmy plate is a thin plate that is used to measure equilibrium surface or interfacial tension at an air–liquid or liquid–liquid interface. In this method, the plate is oriented perpendicular to the interface, and the force exerted on ...
or the Du Noüy ring method
In surface science, the du Noüy ring method is a technique for measuring the surface tension of a liquid. The method involves slowly lifting a ring, often made of platinum, from the surface of a liquid. The force, , required to raise the ring ...
to predict if the adhesive will make good contact with the membrane.
Consolidation stage
Strong and prolonged adhesion
 The consolidation stage of mucoadhesion involves the establishment of adhesive interactions to reinforce strong or prolonged adhesion. When moisture is present, mucoadhesive materials become activated and the system becomes plasticized. This stimulus allows the mucoadhesive molecules to separate and break free while proceeding to link up by weak van der Waals and
The consolidation stage of mucoadhesion involves the establishment of adhesive interactions to reinforce strong or prolonged adhesion. When moisture is present, mucoadhesive materials become activated and the system becomes plasticized. This stimulus allows the mucoadhesive molecules to separate and break free while proceeding to link up by weak van der Waals and hydrogen bonds
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
. Consolidation factors are essential for the surface when exposed to significant dislodging stresses. Multiple mucoadhesion theories exist that explain the consolidation stage, the main two which focus on macromolecular interpenetration and dehydration.
Macromolecular interpenetration theory
 The Macromolecular Interpenetration theory, also known as the diffusion theory, states that the mucoadhesive molecules and mucus glycoproteins mutually interact by means of interpenetration of their chains and the forming of secondary semi-permanent adhesive bonds. It is necessary that the mucoadhesive device has features or properties that favor both chemical and mechanical interactions for the macromolecular interpenetration theory to take place. Molecules that can present mucoadhesive properties are molecules with hydrogen bond building groups, high molecular weight, flexible chains, and surface active properties.
It is perceived that increase in adhesion force is associated with the degree of penetration of polymer chains. Literature states that the degree of penetration required for efficient bioadhesive bonds lies in the range of 0.2-0.5μm. The following equation can be used to estimate the degree of penetration of polymer and mucus chains:
with as contact time and as the
The Macromolecular Interpenetration theory, also known as the diffusion theory, states that the mucoadhesive molecules and mucus glycoproteins mutually interact by means of interpenetration of their chains and the forming of secondary semi-permanent adhesive bonds. It is necessary that the mucoadhesive device has features or properties that favor both chemical and mechanical interactions for the macromolecular interpenetration theory to take place. Molecules that can present mucoadhesive properties are molecules with hydrogen bond building groups, high molecular weight, flexible chains, and surface active properties.
It is perceived that increase in adhesion force is associated with the degree of penetration of polymer chains. Literature states that the degree of penetration required for efficient bioadhesive bonds lies in the range of 0.2-0.5μm. The following equation can be used to estimate the degree of penetration of polymer and mucus chains:
with as contact time and as the diffusion coefficient
Diffusivity, mass diffusivity or diffusion coefficient is a proportionality constant between the molar flux due to molecular diffusion and the gradient in the concentration of the species (or the driving force for diffusion). Diffusivity is enco ...
of the mucoadhesive material in the mucus. Maximum adhesion strength is reached when penetration depth is approximately equal to polymer chain size. Properties of mutual solubility
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubil ...
and structural similarity will improve the mucoadhesive bond.
Dehydration theory
The dehydration theory explains why mucoadhesion can arise rapidly. When two gels capable of rapid gelation in an aqueous environment are brought into contact, movement occurs between the two gels until a state of equilibrium is reached. Gels associated with a strong affinity for water will have high osmotic pressures and large swelling forces. The difference in osmotic pressure when these gels contact mucus gels will draw water into the formulation and quickly dehydrate the mucus gel, forcing intermixing and consolidation until equilibrium results. This mixture of formulation and mucus can increase contact time with the mucous membrane, leading to the consolidation of the adhesive bond. However, the dehydration theory does not apply to solid formulations or highly hydrated forms.Mucoadhesives in drug delivery
Depending on the dosage form androute of administration
A route of administration in pharmacology and toxicology is the way by which a medication, drug, fluid, poison, or other substance is taken into the body.
Routes of administration are generally classified by the location at which the substance i ...
, mucoadhesives may be used for either local or systemic drug delivery
Drug delivery refers to approaches, formulations, manufacturing techniques, storage systems, and technologies involved in transporting a pharmaceutical compound to its target site to achieve a desired therapeutic effect. Principles related to d ...
. An overview on the mucoadhesive properties of mucoadhesives is provided by Vjera Grabovac and Andreas Bernkop-Schnürch. The bioavailability
In pharmacology, bioavailability is a subcategory of absorption and is the fraction (%) of an administered drug that reaches the systemic circulation.
By definition, when a medication is administered intravenously, its bioavailability is 100%. H ...
of such drugs is affected by many factors unique to each route of application. In general, mucoadhesives work to increase the contact time at these sites, prolonging the residence time
The residence time of a fluid parcel is the total time that the parcel has spent inside a control volume (e.g.: a chemical reactor, a lake, a human body). The residence time of a set of parcels is quantified in terms of the frequency distribution ...
and maintaining an effective release rate. These polymeric coatings may be applied to a wide variety of liquid and solid dosages, each specially suited for the route of administration.
Dosage Forms


Tablets
Tablets are small, solid dosages suitable for the use of mucoadhesive coatings. The coating may be formulated to adhere to a specific mucosa, enabling both systemic and targeted local administration. Tablets are generally taken enterally, as the size and stiffness of the form results in poor patient compliance when administered through other routes.Patches
In general, patches consist of three separate layers that contribute and control the release of medicine. The outer impermeable backing layer controls the direction of release and reduces drug loss away from the site of contact. It also protects the other layers and acts as a mechanical support. The middle reservoir layer holds the drug and is tailored to provide the specified dosage. The final inner layer consists of the mucoadhesive, allowing the patch to adhere to the specified mucosa.Gels
As a liquid or semisolid dosage,gel
A gel is a semi-solid that can have properties ranging from soft and weak to hard and tough. Gels are defined as a substantially dilute cross-linked system, which exhibits no flow when in the steady-state, although the liquid phase may still dif ...
s are typically used where a solid form would affect the patient’s comfort. As a trade-off, conventional gels have poor retention rates. This results in unpredictable losses of the drug, as the non-solid dosage is unable to maintain its position at the site of administration. Mucoadhesives increase retention by dynamically increasing the viscosity of the gel after application. This allows the gel to effectively administer the drug at the local site while maintaining the comfort of the patient.
Solutions
These dosage forms are commonly used to deliver drugs to the eye and nasal cavity. They often include mucoadhesive polymers to improve retention on dynamic mucosal surfaces. Some advanced eye drop formulations may also turn from a liquid to a gel (so called in situ gelling systems) upon drug administration. For example, gel-forming solutions containing Pluronics could be used to improve the efficiency of eye drops and provide better retention on ocular surfaces.Routes of Administration
Oromucosal
With a 0.1-0.7 mm thick mucus layer, the oral cavity serves as an important route of administration for mucoadhesive dosages. Permeation sites can be separated into two groups:sublingual
Sublingual (abbreviated SL), from the Latin for "under the tongue", refers to the pharmacological route of administration by which substances diffuse into the blood through tissues under the tongue.
The sublingual glands receive their prima ...
and buccal, in which the former is much more permeable than the latter. However, the sublingual mucosa also produces more saliva
Saliva (commonly referred to as spit) is an extracellular fluid produced and secreted by salivary glands in the mouth. In humans, saliva is around 99% water, plus electrolytes, mucus, white blood cells, epithelial cells (from which DNA can be ...
, resulting in relatively low retention rates. Thus, sublingual mucosa is preferable for rapid onset and short duration treatments, while the buccal mucosa is more appropriate for longer dosage and onset times. Because of this dichotomy, the oral cavity is suitable for both local and systemic administration. Some common dosage forms for the oral cavity include gels, ointments, patches, and tablets. Depending on the dosage form, some drug loss can occur due to swallowing of saliva. This can be minimized by layering the side of the dosage facing the oral cavity with an impermeable coating(,) commonly seen in patches.
Nasal
With an active surface area of 160 cm2, thenasal cavity
The nasal cavity is a large, air-filled space above and behind the nose in the middle of the face. The nasal septum divides the cavity into two cavities, also known as fossae. Each cavity is the continuation of one of the two nostrils. The nasal c ...
is another noteworthy route of mucoadhesive administration. Due to the sweeping motion of the cilia
The cilium, plural cilia (), is a membrane-bound organelle found on most types of eukaryotic cell, and certain microorganisms known as ciliates. Cilia are absent in bacteria and archaea. The cilium has the shape of a slender threadlike projecti ...
that lines the mucosa, nasal mucus has a quick turnover of 10 to 15 minutes. Because of this, the nasal cavity is most suitable for rapid, local medicinal dosages. Additionally, its close proximity to the blood–brain barrier
The blood–brain barrier (BBB) is a highly selective semipermeable membrane, semipermeable border of endothelium, endothelial cells that prevents solutes in the circulating blood from ''non-selectively'' crossing into the extracellular fluid of ...
makes it a convenient route for administering specialized drugs to the central nervous system. Gels, solutions, and aerosols are common dosage forms in the nasal cavity. However, recent research into particles and microspheres have shown increased bioavailability over non-solid forms of medicine largely due to the use of mucoadhesives.
Ocular
Within theeye
Eyes are organs of the visual system. They provide living organisms with vision, the ability to receive and process visual detail, as well as enabling several photo response functions that are independent of vision. Eyes detect light and conv ...
, it is difficult to achieve therapeutic concentrations through systemic administration. Often, other parts of the body will reach toxic levels of the medication before the eye reaches the treatment concentration. Consequently, direct administration through the fibrous tunic is common. This is made difficult due to the numerous defense mechanisms in place, such as blink
Blinking is a bodily function; it is a semi-autonomic rapid closing of the eyelid. A single blink is determined by the forceful closing of the eyelid or inactivation of the levator palpebrae superioris and the activation of the palpebral portio ...
ing, tear production, and the tightness of the corneal epithelium
The corneal epithelium (epithelium corneæ anterior layer) is made up of epithelial tissue and covers the front of the cornea. It acts as a barrier to protect the cornea, resisting the free flow of fluids from the tears, and prevents bacteria fro ...
. Estimates put tear turnover rates at 5 minutes, meaning most conventional drugs are not retained for long periods of time. Mucoadhesives increase retention rates, either by enhancing the viscosity or bonding directly to one of the mucosae surrounding the eye.
Intravesical
Intravesical drug administration is the delivery of pharmaceuticals to the urinary bladder through a catheter. This route of administration is used for the therapy of bladder cancer and interstitial cystitis. The retention of dosage forms in the bladder is relatively poor, which is related to the need for a periodical urine voiding. Some mucoadhesive materials are able to stick to mucosal lining in the bladder, resist urine wash out effects and provide a sustained drug delivery.See also
*Bioadhesives Bioadhesives are natural polymeric materials that act as adhesives. The term is sometimes used more loosely to describe a glue formed synthetically from biological monomers such as sugars, or to mean a synthetic material designed to adhere to biolo ...
* Thiomer
* Wetting
Wetting is the ability of a liquid to maintain contact with a solid surface, resulting from intermolecular interactions when the two are brought together. This happens in presence of a gaseous phase or another liquid phase not miscible with th ...
* Adsorption
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a f ...
* DLVO theory
The DLVO theory (named after Boris Derjaguin and Lev Landau, Evert Verwey and Theodoor Overbeek) explains the aggregation of aqueous dispersions quantitatively and describes the force between charged surfaces interacting through a liquid medium.
...
References
{{Reflist Adhesives Biomolecules Body fluids Excretion Routes of administration