Mitsunobu reaction on:
[Wikipedia]
[Google]
[Amazon]
The Mitsunobu reaction is an  Several reviews have been published.
Several reviews have been published.
 Hughes ''et al.'' have found that the formation of the ion pair 5 is very fast. The formation of the oxyphosphonium intermediate 8 is slow and facilitated by the alkoxide. Therefore, the overall rate of reaction is controlled by carboxylate basicity and solvation.
Hughes ''et al.'' have found that the formation of the ion pair 5 is very fast. The formation of the oxyphosphonium intermediate 8 is slow and facilitated by the alkoxide. Therefore, the overall rate of reaction is controlled by carboxylate basicity and solvation.
 Tsunoda ''et al.'' have shown that one can combine the triphenylphosphine and the diethyl azodicarboxylate into one reagent: a phosphorane
Tsunoda ''et al.'' have shown that one can combine the triphenylphosphine and the diethyl azodicarboxylate into one reagent: a phosphorane  The ylide acts as both the reducing agent and the base. The byproducts are
The ylide acts as both the reducing agent and the base. The byproducts are
 With these particular reactants the conversion with DEAD fails because the
With these particular reactants the conversion with DEAD fails because the Mitsunobu Reaction at SynArchive
Accessed April 26, 2014
organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical ...
that converts an alcohol into a variety of functional groups, such as an ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ...
, using triphenylphosphine
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to P Ph3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists ...
and an azodicarboxylate such as diethyl azodicarboxylate
Diethyl azodicarboxylate, conventionally abbreviated as DEAD and sometimes as DEADCAT, is an organic compound with the structural formula CH3CH2O2CN=NCO2CH2CH3. Its molecular structure consists of a central azo functional group, RN=NR, flanke ...
(DEAD) or diisopropyl azodicarboxylate (DIAD). Although DEAD and DIAD are most commonly used, there are a variety of other azodicarboxylates available which facilitate an easier workup and/or purification and in some cases, facilitate the use of more basic nucleophiles. It was discovered by Oyo Mitsunobu (1934–2003). Typical protocol is to add the phosphine and azodicarboxylate together at −10 °C, typically in THF
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water-miscible organic liquid with low viscosity. It is ma ...
or toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) ...
, until a white precipitate forms. This white, cloudy suspension is the ylide An ylide or ylid () is a neutral dipolar molecule containing a formally negatively charged atom (usually a carbanion) directly attached to a heteroatom with a formal positive charge (usually nitrogen, phosphorus or sulfur), and in which both atoms ...
. Then a solution of the nucleophile and alcohol are added together and the reaction can be, and in many cases is, heated to reflux. The alcohol reacts with the phosphine to create a good leaving group then undergoes an inversion
Inversion or inversions may refer to:
Arts
* , a French gay magazine (1924/1925)
* ''Inversion'' (artwork), a 2005 temporary sculpture in Houston, Texas
* Inversion (music), a term with various meanings in music theory and musical set theory
* ...
of stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereoi ...
in classic SN2 fashion as the nucleophile displaces it. A common side-product is produced when the azodicarboxylate displaces the leaving group instead of the desired nucleophile. This happens if the nucleophile is not acidic enough ( pKa larger than 13) or is not nucleophilic enough due to steric or electronic constraints. A variation of this reaction utilizing a nitrogen nucleophile is known as a Fukuyama–Mitsunobu.
Reaction mechanism
Thereaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage o ...
of the Mitsunobu reaction is fairly complex. The identity of intermediates and the roles they play has been the subject of debate.
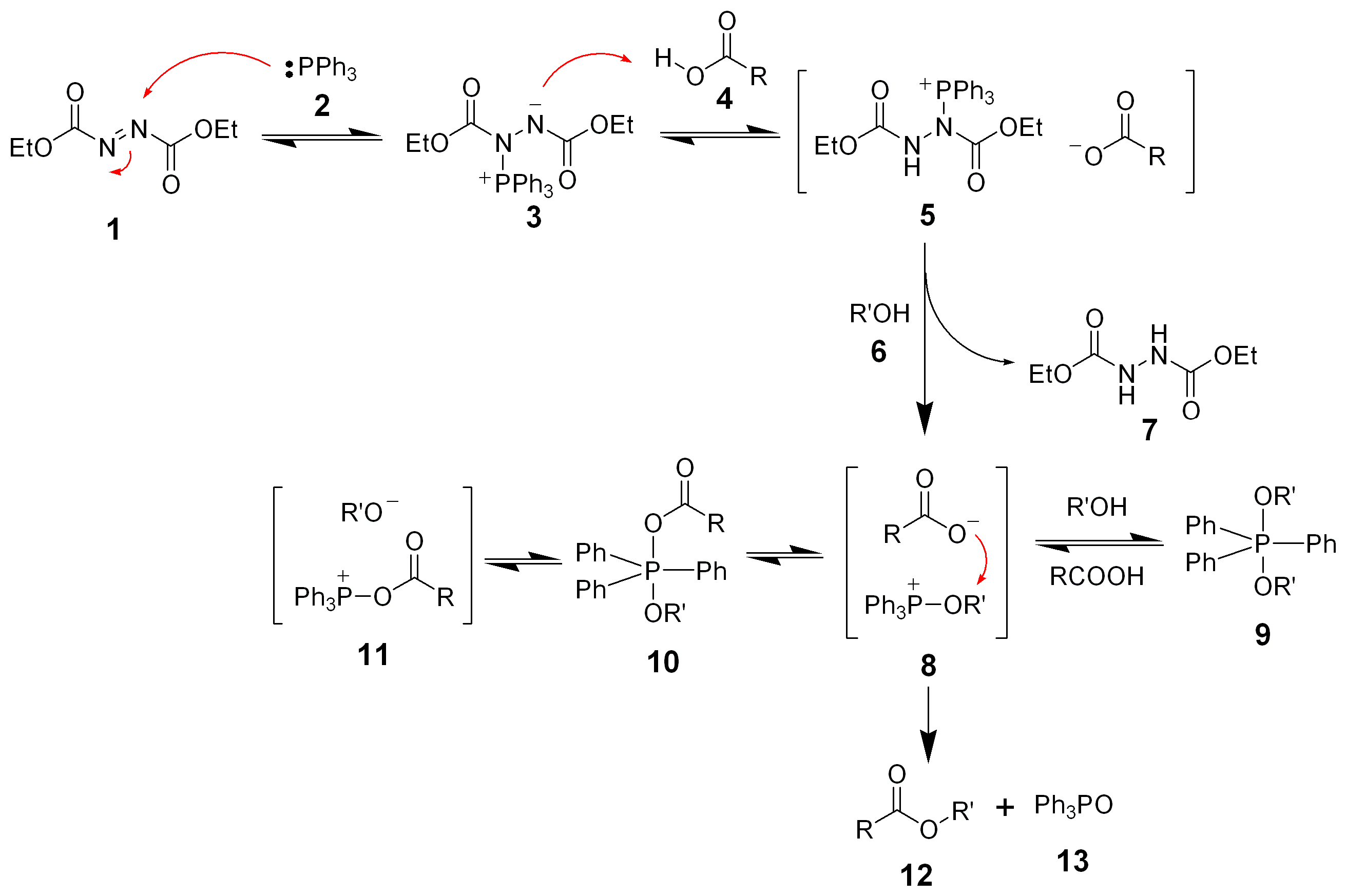
Initially, the triphenyl phosphine (2) makes a nucleophilic attack upon diethyl azodicarboxylate (1) producing a betaine intermediate 3, which deprotonates the carboxylic acid (4) to form the ion pair 5. DEAD itself deprotonates the alcohol (6) forming an alkoxide that can form the key oxyphosphonium ion 8. The ratio and interconversion of intermediates 8–11 depend on the carboxylic acid pKa and the solvent polarity. Although several phosphorus intermediates are present, the attack of the carboxylate anion upon intermediate 8 is the only productive pathway forming the desired product 12 and triphenylphosphine oxide (13).
 Hughes ''et al.'' have found that the formation of the ion pair 5 is very fast. The formation of the oxyphosphonium intermediate 8 is slow and facilitated by the alkoxide. Therefore, the overall rate of reaction is controlled by carboxylate basicity and solvation.
Hughes ''et al.'' have found that the formation of the ion pair 5 is very fast. The formation of the oxyphosphonium intermediate 8 is slow and facilitated by the alkoxide. Therefore, the overall rate of reaction is controlled by carboxylate basicity and solvation.
Order of addition of reagents
The order of addition of the reagents of the Mitsunobu reaction can be important. Typically, one dissolves the alcohol, the carboxylic acid, and triphenylphosphine intetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
or other suitable solvent (e.g. diethyl ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable li ...
), cool to 0 °C using an ice-bath, slowly add the DEAD dissolved in THF, then stir at room temperature for several hours. If this is unsuccessful, then preforming the betaine may give better results. To preform the betaine, add DEAD to triphenylphosphine in tetrahydrofuran at 0 °C, followed by the addition of the alcohol and finally the acid.
Variations
Other nucleophilic functional groups
Many other functional groups can serve asnucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they ar ...
s besides carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxyli ...
s. For the reaction to be successful, the nucleophile must have a pKa less than 15.
Modifications
Several modifications to the original reagent combination have been developed in order to simplify the separation of the product and avoid production of so much chemical waste. One variation of the Mitsunobu reaction uses resin-bound triphenylphosphine and uses di-''tert''-butylazodicarboxylate instead of DEAD. The oxidized triphenylphosphine resin can be removed by filtration, and the di-''tert''-butylazodicarboxylate byproduct is removed by treatment withtrifluoroacetic acid
Trifluoroacetic acid (TFA) is an organofluorine compound with the chemical formula CF3CO2H. It is a structural analogue of acetic acid with all three of the acetyl group's hydrogen atoms replaced by fluorine atoms and is a colorless liquid with ...
. Bruce H. Lipshutz Bruce H. Lipshutz (born 1951) is an American chemist. He is a professor at the University of California, Santa Barbara.
Biography
Lipshutz received his undergraduate degree in chemistry from Binghamton University in 1973. His graduate work was supe ...
has developed an alternative to DEAD, di-(4-chlorobenzyl)azodicarboxylate (DCAD) where the hydrazine by-product can be easily removed by filtration and recycled back to DCAD.
A modification has also been reported in which DEAD can be used in catalytic versus stoichiometric quantities, however this procedure requires the use of stoichiometric (diacetoxyiodo)benzene to oxidise the hydrazine by-product back to DEAD.
Denton and co-workers have reported a redox-neutral variant of the Mitsunobu reaction which employs a phosphorus(III) catalyst to activate the substrate, ensuring inversion in the nucleophilic attack, and uses a Dean-Stark trap to remove the water by-product.
Phosphorane reagents
ylide An ylide or ylid () is a neutral dipolar molecule containing a formally negatively charged atom (usually a carbanion) directly attached to a heteroatom with a formal positive charge (usually nitrogen, phosphorus or sulfur), and in which both atoms ...
. Both (cyanomethylene)trimethylphosphorane (CMMP, R = Me) and (cyanomethylene)tributylphosphorane (CMBP, R = Bu) have proven particularly effective.
 The ylide acts as both the reducing agent and the base. The byproducts are
The ylide acts as both the reducing agent and the base. The byproducts are acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile ( hydrogen cyanide is a simpler nitrile, but the cyanide anion is not clas ...
(6) and the trialkylphosphine oxide (8).
Uses
The Mitsunobu reaction has been applied in the synthesis ofaryl ether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be c ...
s:
 With these particular reactants the conversion with DEAD fails because the
With these particular reactants the conversion with DEAD fails because the hydroxyl group
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
is only weakly acidic. Instead the related ''1,1'-(azodicarbonyl)dipiperidine'' (ADDP) is used of which the betaine intermediate is a stronger base. The phosphine is a polymer-supported triphenylphosphine (PS-PPh3).
The reaction has been used to synthesize quinine
Quinine is a medication used to treat malaria and babesiosis. This includes the treatment of malaria due to '' Plasmodium falciparum'' that is resistant to chloroquine when artesunate is not available. While sometimes used for nocturnal leg ...
, colchicine
Colchicine is a medication used to treat gout and Behçet's disease. In gout, it is less preferred to NSAIDs or steroids. Other uses for colchicine include the management of pericarditis and familial Mediterranean fever. Colchicine is taken b ...
, sarain, morphine
Morphine is a strong opiate that is found naturally in opium, a dark brown resin in poppies ('' Papaver somniferum''). It is mainly used as a pain medication, and is also commonly used recreationally, or to make other illicit opioids. T ...
, stigmatellin, eudistomin, oseltamivir, strychnine
Strychnine (, , US chiefly ) is a highly toxic, colorless, bitter, crystalline alkaloid used as a pesticide, particularly for killing small vertebrates such as birds and rodents. Strychnine, when inhaled, swallowed, or absorbed through the e ...
, and nupharamine.Accessed April 26, 2014
References
See also
* Appel reaction {{DEFAULTSORT:Mitsunobu reaction Substitution reactions Name reactions