MeV on:
[Wikipedia]
[Google]
[Amazon]
In

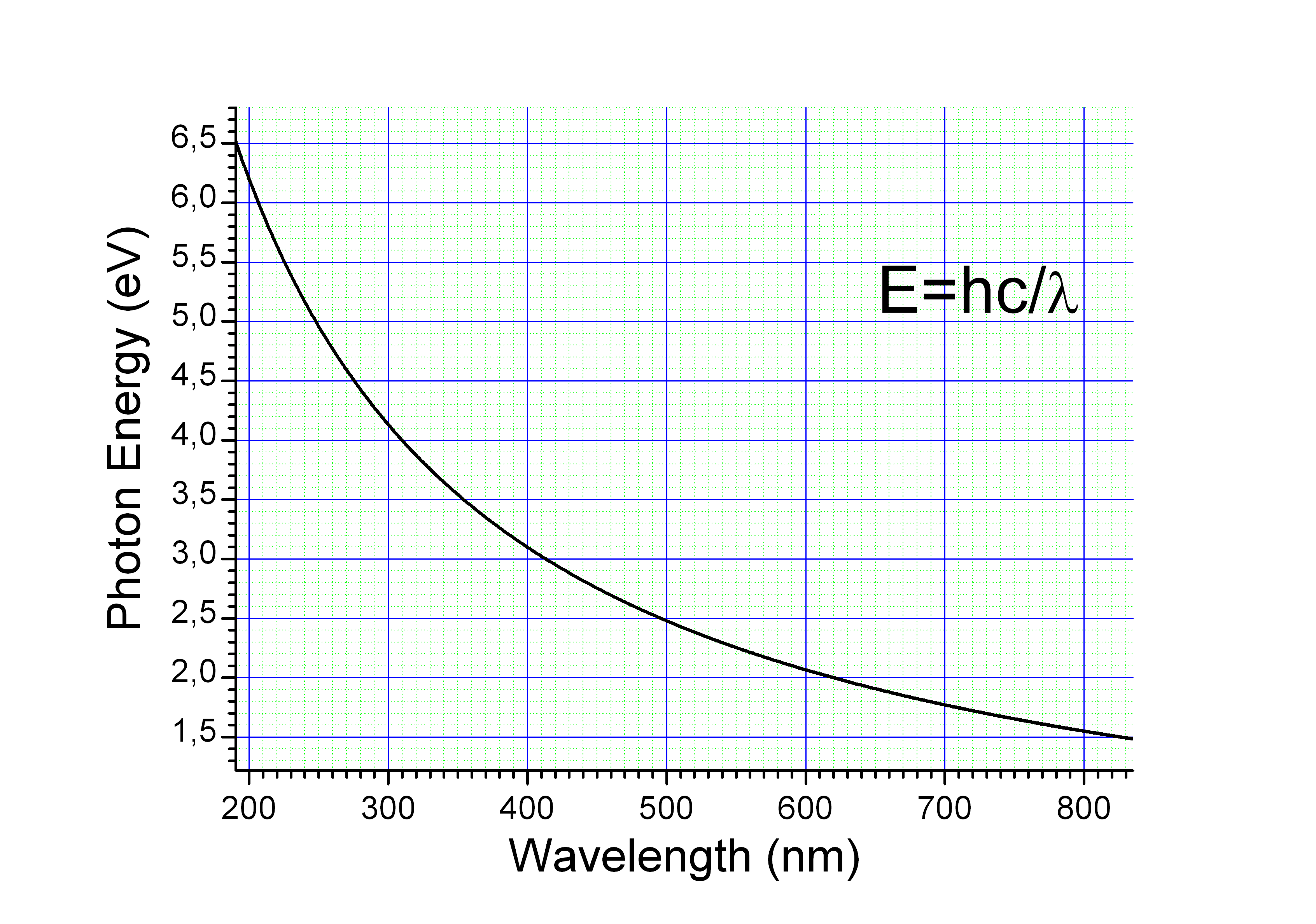 The energy ''E'', frequency ''v'', and wavelength ''λ'' of a photon are related by
where ''h'' is the Planck constant, ''c'' is the speed of light. This reduces to
A photon with a wavelength of (green light) would have an energy of approximately . Similarly, would correspond to an infrared photon of wavelength or frequency .
The energy ''E'', frequency ''v'', and wavelength ''λ'' of a photon are related by
where ''h'' is the Planck constant, ''c'' is the speed of light. This reduces to
A photon with a wavelength of (green light) would have an energy of approximately . Similarly, would correspond to an infrared photon of wavelength or frequency .

BIPM's definition of the electronvoltphysical constants reference; CODATA data
{{DEFAULTSORT:Electron Volt Particle physics Units of chemical measurement Units of energy Voltage
physics
Physics is the natural science that studies matter, its fundamental constituents, its motion and behavior through space and time, and the related entities of energy and force. "Physical science is that department of knowledge which ...
, an electronvolt (symbol eV, also written electron-volt and electron volt) is the measure of an amount of kinetic energy gained by a single electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have n ...
accelerating from rest through an electric potential difference of one volt in vacuum
A vacuum is a space devoid of matter. The word is derived from the Latin adjective ''vacuus'' for "vacant" or " void". An approximation to such vacuum is a region with a gaseous pressure much less than atmospheric pressure. Physicists often ...
. When used as a unit of energy, the numerical value of 1 eV in joule
The joule ( , ; symbol: J) is the unit of energy in the International System of Units (SI). It is equal to the amount of work done when a force of 1 newton displaces a mass through a distance of 1 metre in the direction of the force appli ...
s (symbol J) is equivalent to the numerical value of the charge of an electron in coulombs (symbol C). Under the 2019 redefinition of the SI base units, this sets 1 eV equal to the exact value
Historically, the electronvolt was devised as a standard unit of measure through its usefulness in electrostatic particle accelerator sciences, because a particle with electric charge
Electric charge is the physical property of matter that causes charged matter to experience a force when placed in an electromagnetic field. Electric charge can be ''positive'' or ''negative'' (commonly carried by protons and electrons res ...
''q'' gains an energy after passing through a voltage of ''V.'' Since ''q'' must be an integer multiple of the elementary charge ''e'' for any isolated particle, the gained energy in units of electronvolts conveniently equals that integer times the voltage.
It is a common unit of energy within physics, widely used in solid state, atomic, nuclear
Nuclear may refer to:
Physics
Relating to the nucleus of the atom:
*Nuclear engineering
*Nuclear physics
*Nuclear power
*Nuclear reactor
*Nuclear weapon
*Nuclear medicine
*Radiation therapy
*Nuclear warfare
Mathematics
*Nuclear space
*Nuclear ...
, and particle physics, and high-energy astrophysics High energy astronomy is the study of astronomical objects that release electromagnetic radiation of highly energetic wavelengths. It includes X-ray astronomy, gamma-ray astronomy, extreme UV astronomy, neutrino astronomy, and studies of cosmic ...
. It is commonly used with SI prefixes milli-, kilo-, mega-, giga-, tera-, peta- or exa- (meV, keV, MeV, GeV, TeV, PeV and EeV respectively). In some older documents, and in the name Bevatron, the symbol BeV is used, which stands for billion (109) electronvolts; it is equivalent to the GeV.
Definition
An electronvolt is the amount of kinetic energy gained or lost by a singleelectron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have n ...
accelerating from rest through an electric potential difference of one volt in vacuum. Hence, it has a value of one volt, , multiplied by the elementary charge Therefore, one electronvolt is equal to
The electronvolt (eV) is a unit of energy, but is not an SI unit. The SI unit of energy is the joule (J).
Relation to other physical properties and units
Mass
By mass–energy equivalence, the electronvolt corresponds to a unit ofmass
Mass is an intrinsic property of a body. It was traditionally believed to be related to the quantity of matter in a physical body, until the discovery of the atom and particle physics. It was found that different atoms and different ele ...
. It is common in particle physics, where units of mass and energy are often interchanged, to express mass in units of eV/''c''2, where ''c'' is the speed of light in vacuum (from ). It is common to informally express mass in terms of eV as a unit of mass
Mass is an intrinsic property of a body. It was traditionally believed to be related to the quantity of matter in a physical body, until the discovery of the atom and particle physics. It was found that different atoms and different elementar ...
, effectively using a system of natural units with ''c'' set to 1. The kilogram equivalent of is:
For example, an electron and a positron, each with a mass of , can annihilate to yield of energy. A proton has a mass of . In general, the masses of all hadrons are of the order of , which makes the GeV/''c''2 a convenient unit of mass for particle physics:
The atomic mass constant (''m''u), one twelfth of the mass a carbon-12 atom, is close to the mass of a proton. To convert to electronvolt mass-equivalent, use the formula:
Momentum
By dividing a particle's kinetic energy in electronvolts by the fundamental constant ''c'' (the speed of light), one can describe the particle's momentum in units of eV/''c''. In natural units in which the fundamental velocity constant ''c'' is numerically 1, the ''c'' may informally be omitted to express momentum as electronvolts. The energy momentum relation in natural units (with ) is a Pythagorean equation. When a relatively high energy is applied to a particle with relatively low rest mass, it can be approximated as in high-energy physics such that an applied energy in units of eV conveniently results in an approximately equivalent change of momentum in units of eV/''c''. The dimensions of momentum units are . The dimensions of energy units are . Dividing the units of energy (such as eV) by a fundamental constant (such as the speed of light) that has units of velocity () facilitates the required conversion for using energy units to describe momentum. For example, if the momentum ''p'' of an electron is said to be , then the conversion toMKS system of units
The MKS system of units is a physical system of measurement that uses the metre, kilogram, and second (MKS) as base units. It forms the base of the International System of Units (SI), though SI has since been redefined by different fundament ...
can be achieved by:
Distance
In particle physics, a system of natural units in which the speed of light in vacuum ''c'' and the reduced Planck constant ''ħ'' are dimensionless and equal to unity is widely used: . In these units, both distances and times are expressed in inverse energy units (while energy and mass are expressed in the same units, see mass–energy equivalence). In particular, particle scattering lengths are often presented in units of inverse particle masses. Outside this system of units, the conversion factors between electronvolt, second, and nanometer are the following: : The above relations also allow expressing the mean lifetime ''τ'' of an unstable particle (in seconds) in terms of its decay width Γ (in eV) via . For example, the meson has a lifetime of 1.530(9) picoseconds, mean decay length is , or a decay width of . Conversely, the tiny meson mass differences responsible for meson oscillations are often expressed in the more convenient inverse picoseconds. Energy in electronvolts is sometimes expressed through the wavelength of light with photons of the same energy: :Temperature
In certain fields, such as plasma physics, it is convenient to use the electronvolt to express temperature. The electronvolt is divided by theBoltzmann constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin and the gas constan ...
to convert to the Kelvin scale
The kelvin, symbol K, is the primary unit of temperature in the International System of Units (SI), used alongside its prefixed forms and the degree Celsius. It is named after the Belfast-born and University of Glasgow-based engineer and p ...
:
:
where ''k''B is the Boltzmann constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin and the gas constan ...
.
The ''k''B is assumed when using the electronvolt to express temperature, for example, a typical magnetic confinement fusion plasma is (kiloelectronvolt), which is equal to 174 MK (megakelvin).
As an approximation: ''k''B''T'' is about (≈ ) at a temperature of .
Wavelength
 The energy ''E'', frequency ''v'', and wavelength ''λ'' of a photon are related by
where ''h'' is the Planck constant, ''c'' is the speed of light. This reduces to
A photon with a wavelength of (green light) would have an energy of approximately . Similarly, would correspond to an infrared photon of wavelength or frequency .
The energy ''E'', frequency ''v'', and wavelength ''λ'' of a photon are related by
where ''h'' is the Planck constant, ''c'' is the speed of light. This reduces to
A photon with a wavelength of (green light) would have an energy of approximately . Similarly, would correspond to an infrared photon of wavelength or frequency .
Scattering experiments
In a low-energy nuclear scattering experiment, it is conventional to refer to the nuclear recoil energy in units of eVr, keVr, etc. This distinguishes the nuclear recoil energy from the "electron equivalent" recoil energy (eVee, keVee, etc.) measured by scintillation light. For example, the yield of a phototube is measured in phe/keVee ( photoelectrons per keV electron-equivalent energy). The relationship between eV, eVr, and eVee depends on the medium the scattering takes place in, and must be established empirically for each material.Energy comparisons
Per mole
One mole of particles given 1 eV of energy each has approximately 96.5 kJ of energy – this corresponds to the Faraday constant (''F'' ≈ ), where the energy in joules of ''n'' moles of particles each with energy ''E'' eV is equal to ''E''·''F''·''n''.See also
* Orders of magnitude (energy)References
External links
BIPM's definition of the electronvolt
{{DEFAULTSORT:Electron Volt Particle physics Units of chemical measurement Units of energy Voltage