Myers' Asymmetric Alkylation on:
[Wikipedia]
[Google]
[Amazon]
In 
Most biological molecules and pharmaceutical targets exist as one of two possible
 A typical auxiliary-guided stereoselective transformation involves three steps: first, the auxiliary is covalently coupled to the substrate; second, the resulting compound undergoes one or more diastereoselective transformations; and finally, the auxiliary is removed under conditions that do not cause racemization of the desired products. The cost of employing stoichiometric auxiliary and the need to spend synthetic steps appending and removing the auxiliary make this approach appear inefficient. However, for many transformations, the only available stereoselective methodology relies on chiral auxiliaries. In addition, transformations with chiral auxiliaries tend to be versatile and very well-studied, allowing the most time-efficient access to enantiomerically pure products.
Furthermore, the products of auxiliary-directed reactions are diastereomers, which enables their facile separation by methods such as column chromatography or crystallization.
A typical auxiliary-guided stereoselective transformation involves three steps: first, the auxiliary is covalently coupled to the substrate; second, the resulting compound undergoes one or more diastereoselective transformations; and finally, the auxiliary is removed under conditions that do not cause racemization of the desired products. The cost of employing stoichiometric auxiliary and the need to spend synthetic steps appending and removing the auxiliary make this approach appear inefficient. However, for many transformations, the only available stereoselective methodology relies on chiral auxiliaries. In addition, transformations with chiral auxiliaries tend to be versatile and very well-studied, allowing the most time-efficient access to enantiomerically pure products.
Furthermore, the products of auxiliary-directed reactions are diastereomers, which enables their facile separation by methods such as column chromatography or crystallization.
 (−)-8-phenylmenthol can be prepared from either
(−)-8-phenylmenthol can be prepared from either
 Hisashi Yamamoto first utilized (''R'')-BINOL as a chiral auxiliary in the asymmetric synthesis of limonene, which is an example of cyclic mono- terpenes. (''R'')-BINOL mononeryl ether was prepared by the monosilylation and alkylation of (''R'')-BINOL as the chiral auxiliary. Followed with the reduction by organoaluminum reagent, limonene was synthesized with low yields (29% yield) and moderate enantiomeric excesses up to 64% ee.
Hisashi Yamamoto first utilized (''R'')-BINOL as a chiral auxiliary in the asymmetric synthesis of limonene, which is an example of cyclic mono- terpenes. (''R'')-BINOL mononeryl ether was prepared by the monosilylation and alkylation of (''R'')-BINOL as the chiral auxiliary. Followed with the reduction by organoaluminum reagent, limonene was synthesized with low yields (29% yield) and moderate enantiomeric excesses up to 64% ee.
 The preparation of a variety of enantiomerically pure uncommon ''R''-amino acids can be achieved by the alkylation of chiral glycine derivatives possessing axially chiral BINOL as an auxiliary. It has been depicted by Fuji et al. Based on different electrophile, the diastereomeric excess varied from 69% to 86.
The preparation of a variety of enantiomerically pure uncommon ''R''-amino acids can be achieved by the alkylation of chiral glycine derivatives possessing axially chiral BINOL as an auxiliary. It has been depicted by Fuji et al. Based on different electrophile, the diastereomeric excess varied from 69% to 86.
 Protected at the aldehyde function with (R)-BINOL, arylglyoxals reacted diastereoselectively with Grignard reagents to afford protected atrolactaldehyde with moderate to excellent diastereomeric excess and high yields.
Protected at the aldehyde function with (R)-BINOL, arylglyoxals reacted diastereoselectively with Grignard reagents to afford protected atrolactaldehyde with moderate to excellent diastereomeric excess and high yields.
 BINOL was also used as a chiral auxiliary to control the formation of a P-stereocenter in an asymmetric metal-catalyzed C-P coupling process. Mondal et al. discovered that the Pd-catalysed C-P cross-coupling reaction between axially chiral BINOL-based phosphoramidites and aryl halides or triflates proceeds with excellent stereoselectivity due to the presence of BINOL near the reacting P center.
BINOL was also used as a chiral auxiliary to control the formation of a P-stereocenter in an asymmetric metal-catalyzed C-P coupling process. Mondal et al. discovered that the Pd-catalysed C-P cross-coupling reaction between axially chiral BINOL-based phosphoramidites and aryl halides or triflates proceeds with excellent stereoselectivity due to the presence of BINOL near the reacting P center.
 One type of chiral auxiliary is based on the ''trans''-2-phenylcyclohexanol motif as introduced by James K. Whitesell and coworkers in 1985. This chiral auxiliary was used in ene reactions of the derived ester of glyoxylic acid.
One type of chiral auxiliary is based on the ''trans''-2-phenylcyclohexanol motif as introduced by James K. Whitesell and coworkers in 1985. This chiral auxiliary was used in ene reactions of the derived ester of glyoxylic acid.
 In the total synthesis of (−)-heptemerone B and (−)-guanacastepene E, attached with trans-2-phenylcyclohexanol, the glyoxylate reacted with 2,4-dimethyl-pent-2-ene, in the presence of tin(IV) chloride, yielding the desired anti adduct as the major product, together with a small amount of its syn isomer with 10:1 diastereomeric ratio.
In the total synthesis of (−)-heptemerone B and (−)-guanacastepene E, attached with trans-2-phenylcyclohexanol, the glyoxylate reacted with 2,4-dimethyl-pent-2-ene, in the presence of tin(IV) chloride, yielding the desired anti adduct as the major product, together with a small amount of its syn isomer with 10:1 diastereomeric ratio.
 For even greater conformational control, switching from a phenyl to a trityl group gives ''trans''-2-tritylcyclohexanol (TTC). In 2015, the Brown group published an efficient chiral permanganate-mediated oxidative cyclization with TTC.
For even greater conformational control, switching from a phenyl to a trityl group gives ''trans''-2-tritylcyclohexanol (TTC). In 2015, the Brown group published an efficient chiral permanganate-mediated oxidative cyclization with TTC.

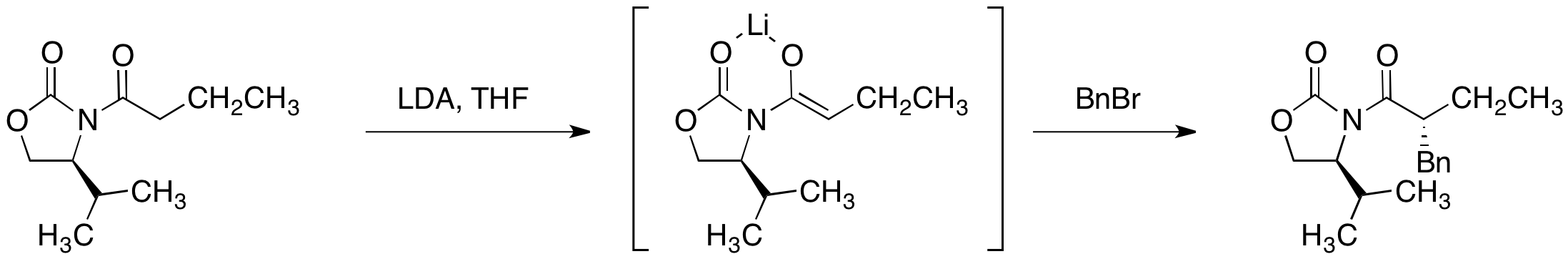
 Acylation of the oxazolidinone is achieved by deprotonation with n-butyllithium and quench with an
Acylation of the oxazolidinone is achieved by deprotonation with n-butyllithium and quench with an 
 Activated electrophiles, such as
Activated electrophiles, such as
 A model for the observed stereoselectivity can be found below. The ''syn''-stereorelationship between the methyl group and the new secondary alcohol results from a six-membered ring Zimmerman-Traxler transition state, wherein the enolate oxygen and the aldheyde oxygen both coordinate boron. The aldehyde is oriented such that the hydrogen is placed in a pseudo-axial orientation to minimize 1,3-diaxial interactions. The absolute stereochemistry of the two stereocenters is controlled by the chirality in the auxiliary. In the transition structure, the auxiliary carbonyl is oriented away from the enolate oxygen so as to minimize the net dipole of the molecule; one face of the enolate is blocked by the substituent on the chiral auxiliary.
A model for the observed stereoselectivity can be found below. The ''syn''-stereorelationship between the methyl group and the new secondary alcohol results from a six-membered ring Zimmerman-Traxler transition state, wherein the enolate oxygen and the aldheyde oxygen both coordinate boron. The aldehyde is oriented such that the hydrogen is placed in a pseudo-axial orientation to minimize 1,3-diaxial interactions. The absolute stereochemistry of the two stereocenters is controlled by the chirality in the auxiliary. In the transition structure, the auxiliary carbonyl is oriented away from the enolate oxygen so as to minimize the net dipole of the molecule; one face of the enolate is blocked by the substituent on the chiral auxiliary.


 In the total synthesis of manzacidin B, Ohfune group utilized camphorsultam to construct the core
In the total synthesis of manzacidin B, Ohfune group utilized camphorsultam to construct the core  Camphorsultam also acts as a chiral auxiliary in Michael addition. Lithium base promoted stereoselective Michael addition of thiols to N-mcthacryloylcamphorsultam produced the corresponding addition products in high diastereoselectivity.
Camphorsultam also acts as a chiral auxiliary in Michael addition. Lithium base promoted stereoselective Michael addition of thiols to N-mcthacryloylcamphorsultam produced the corresponding addition products in high diastereoselectivity.
 Camphorsultam was used as a chiral auxiliary for the asymmetric Claisen rearrangement. In the presence of
Camphorsultam was used as a chiral auxiliary for the asymmetric Claisen rearrangement. In the presence of 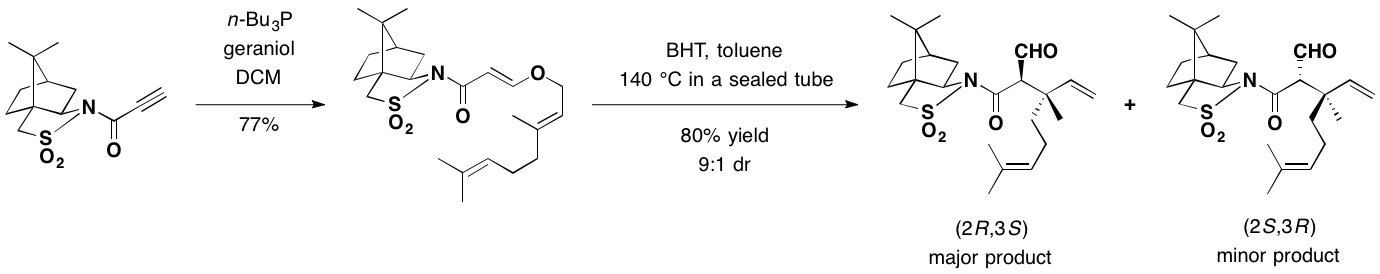
 Pseudoephedrine amides are typically prepared by acylation with an acyl chloride or anhydride.
Pseudoephedrine amides are typically prepared by acylation with an acyl chloride or anhydride.

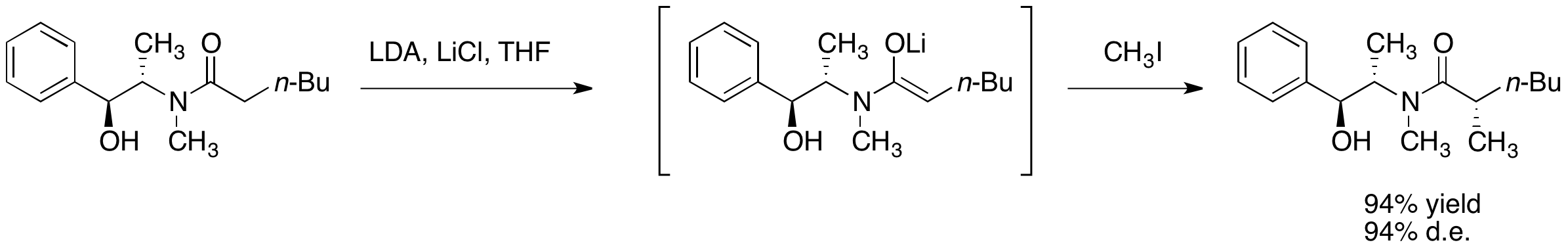
The diastereoselectivity is believed to result from a configuration wherein one face of the lithium enolate is blocked by the secondary lithium alkoxide and the solvent molecules associated with that lithium cation. In accordance with this proposal, it has been observed that the diastereoselctivity of the alkylation step is highly dependent on the amount of lithium chloride present and on the solvent, tetrahydrofuran (THF). Typically, 4 to 6 equivalents of lithium chloride are sufficient to saturate a solution of enolate in THF at the reaction molarity.
One primary advantage of asymmetric alkylation with pseudoephedrine amides is that the amide enolates are typically nucleophilic enough to react with primary and even secondary halides at temperatures ranging from –78 °C to 0 °C. Construction of quaternary carbon centers by alkylation of α-branched amide enolates is also possible, though the addition of



Condensation of ''tert''-butanesulfinamide with an aldehyde or ketone proceeds in high yield and affords only the (''E'')-isomer of the corresponding ''N''-sulfinyl imines.



stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereois ...
, a chiral auxiliary is a stereogenic group or unit that is temporarily incorporated into an organic compound in order to control the stereochemical outcome of the synthesis. The chirality
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from ...
present in the auxiliary can bias the stereoselectivity of one or more subsequent reactions. The auxiliary can then be typically recovered for future use.

Most biological molecules and pharmaceutical targets exist as one of two possible
enantiomers
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical anti ...
; consequently, chemical syntheses of natural products and pharmaceutical agents are frequently designed to obtain the target in enantiomerically pure form. Chiral auxiliaries are one of many strategies available to synthetic chemists to selectively produce the desired stereoisomer of a given compound.
Chiral auxiliaries were introduced by Elias James Corey in 1975 with chiral 8-phenylmenthol and by Barry Trost
Barry M. Trost (born June 13, 1941, in Philadelphia) is an American chemist who is the Job and Gertrud Tamaki Professor Emeritus in the School of Humanities and Sciences at Stanford University. The Tsuji-Trost reaction and the Trost ligand are ...
in 1980 with chiral mandelic acid. The menthol compound is difficult to prepare and as an alternative trans-2-phenyl-1-cyclohexanol was introduced by J. K. Whitesell in 1985.
Asymmetric synthesis
Chiral auxiliaries are incorporated into synthetic routes to control the absolute configuration of stereogenic centers.David A. Evans
David A. Evans (January 11, 1941 – April 29, 2022) was an American chemist who was the Abbott and James Lawrence professor of chemistry at Harvard University. He was a prominent figure in the field of organic chemistry and his research focus ...
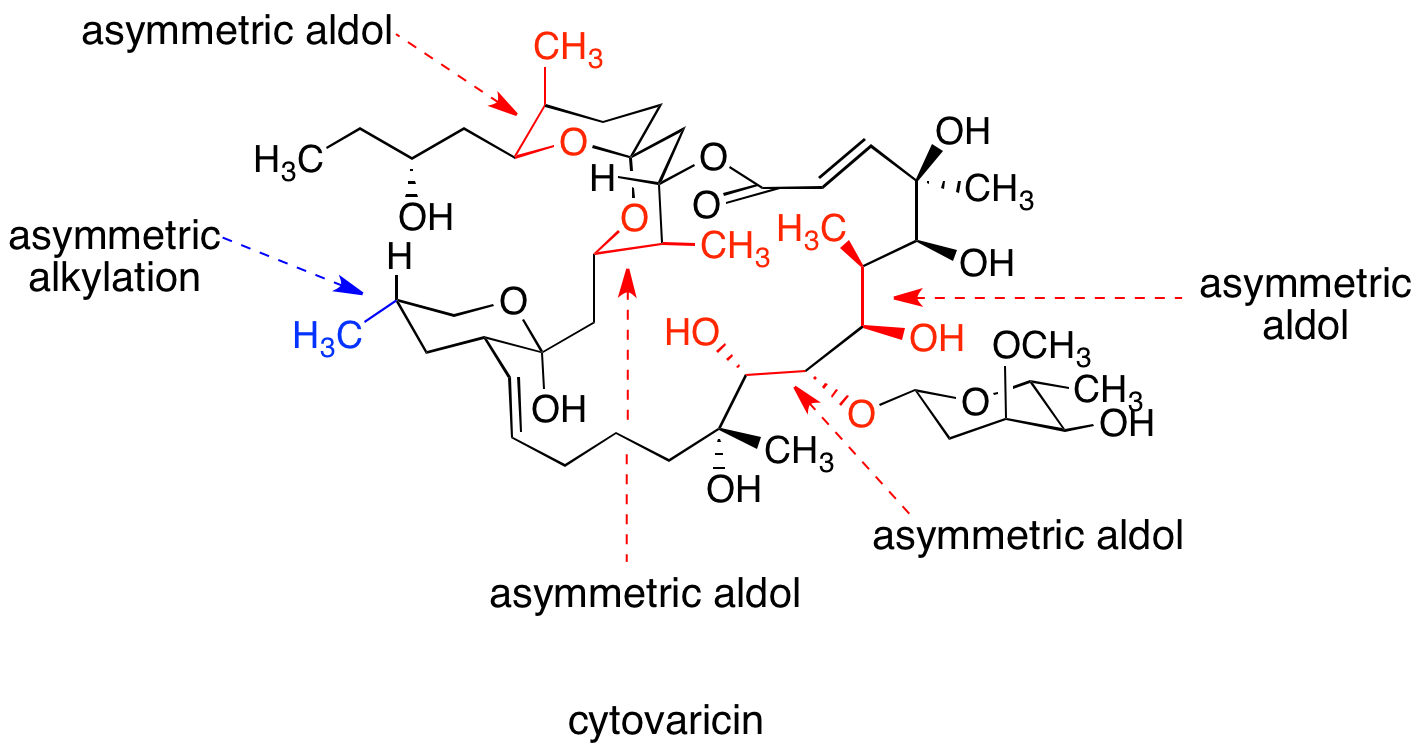
' synthesis of the macrolide cytovaricin, considered a classic, utilizes oxazolidinone chiral auxiliaries for one asymmetric alkylation reaction and four asymmetric aldol reactions, setting the absolute stereochemistry of nine stereocenters.
 A typical auxiliary-guided stereoselective transformation involves three steps: first, the auxiliary is covalently coupled to the substrate; second, the resulting compound undergoes one or more diastereoselective transformations; and finally, the auxiliary is removed under conditions that do not cause racemization of the desired products. The cost of employing stoichiometric auxiliary and the need to spend synthetic steps appending and removing the auxiliary make this approach appear inefficient. However, for many transformations, the only available stereoselective methodology relies on chiral auxiliaries. In addition, transformations with chiral auxiliaries tend to be versatile and very well-studied, allowing the most time-efficient access to enantiomerically pure products.
Furthermore, the products of auxiliary-directed reactions are diastereomers, which enables their facile separation by methods such as column chromatography or crystallization.
A typical auxiliary-guided stereoselective transformation involves three steps: first, the auxiliary is covalently coupled to the substrate; second, the resulting compound undergoes one or more diastereoselective transformations; and finally, the auxiliary is removed under conditions that do not cause racemization of the desired products. The cost of employing stoichiometric auxiliary and the need to spend synthetic steps appending and removing the auxiliary make this approach appear inefficient. However, for many transformations, the only available stereoselective methodology relies on chiral auxiliaries. In addition, transformations with chiral auxiliaries tend to be versatile and very well-studied, allowing the most time-efficient access to enantiomerically pure products.
Furthermore, the products of auxiliary-directed reactions are diastereomers, which enables their facile separation by methods such as column chromatography or crystallization.
8-phenylmenthol
In an early example of the use of a chiral auxiliary in asymmetric synthesis, E. J. Corey and coworkers conducted an asymmetric Diels-Alder reaction between (−)-8-phenylmenthol acrylate ester and 5-benzyloxymethylcyclopentadiene. The cycloaddition product was carried forward to the iodolactone shown below, an intermediate in the classic Corey synthesis of the prostaglandins. It is proposed that the back face of the acrylate is blocked by the auxiliary, so that cycloaddition occurs at the front face of the alkene. (−)-8-phenylmenthol can be prepared from either
(−)-8-phenylmenthol can be prepared from either enantiomer
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical ant ...
of pulegone,
though neither route is very efficient. Because of the widespread utility of the 8-phenylmenthol auxiliary, alternative compounds that are more easily synthesized, such as ''trans''-2-phenyl-1-cyclohexanol
and ''trans''-2-(1-pheyl-1-methylethyl)cyclohexanol have been explored.
1,1’-Binaphthyl-2,2’-diol (BINOL)
1,1’-Binaphthyl-2,2’-diol, or BINOL, has been used as chiral auxiliary for theasymmetric synthesis
Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molecul ...
since 1983.
 The preparation of a variety of enantiomerically pure uncommon ''R''-amino acids can be achieved by the alkylation of chiral glycine derivatives possessing axially chiral BINOL as an auxiliary. It has been depicted by Fuji et al. Based on different electrophile, the diastereomeric excess varied from 69% to 86.
The preparation of a variety of enantiomerically pure uncommon ''R''-amino acids can be achieved by the alkylation of chiral glycine derivatives possessing axially chiral BINOL as an auxiliary. It has been depicted by Fuji et al. Based on different electrophile, the diastereomeric excess varied from 69% to 86.
 Protected at the aldehyde function with (R)-BINOL, arylglyoxals reacted diastereoselectively with Grignard reagents to afford protected atrolactaldehyde with moderate to excellent diastereomeric excess and high yields.
Protected at the aldehyde function with (R)-BINOL, arylglyoxals reacted diastereoselectively with Grignard reagents to afford protected atrolactaldehyde with moderate to excellent diastereomeric excess and high yields.
 BINOL was also used as a chiral auxiliary to control the formation of a P-stereocenter in an asymmetric metal-catalyzed C-P coupling process. Mondal et al. discovered that the Pd-catalysed C-P cross-coupling reaction between axially chiral BINOL-based phosphoramidites and aryl halides or triflates proceeds with excellent stereoselectivity due to the presence of BINOL near the reacting P center.
BINOL was also used as a chiral auxiliary to control the formation of a P-stereocenter in an asymmetric metal-catalyzed C-P coupling process. Mondal et al. discovered that the Pd-catalysed C-P cross-coupling reaction between axially chiral BINOL-based phosphoramidites and aryl halides or triflates proceeds with excellent stereoselectivity due to the presence of BINOL near the reacting P center.
''trans''-2-Phenylcyclohexanol
 In the total synthesis of (−)-heptemerone B and (−)-guanacastepene E, attached with trans-2-phenylcyclohexanol, the glyoxylate reacted with 2,4-dimethyl-pent-2-ene, in the presence of tin(IV) chloride, yielding the desired anti adduct as the major product, together with a small amount of its syn isomer with 10:1 diastereomeric ratio.
In the total synthesis of (−)-heptemerone B and (−)-guanacastepene E, attached with trans-2-phenylcyclohexanol, the glyoxylate reacted with 2,4-dimethyl-pent-2-ene, in the presence of tin(IV) chloride, yielding the desired anti adduct as the major product, together with a small amount of its syn isomer with 10:1 diastereomeric ratio.
 For even greater conformational control, switching from a phenyl to a trityl group gives ''trans''-2-tritylcyclohexanol (TTC). In 2015, the Brown group published an efficient chiral permanganate-mediated oxidative cyclization with TTC.
For even greater conformational control, switching from a phenyl to a trityl group gives ''trans''-2-tritylcyclohexanol (TTC). In 2015, the Brown group published an efficient chiral permanganate-mediated oxidative cyclization with TTC.

Oxazolidinones
Oxazolidinone auxiliaries, popularized byDavid A. Evans
David A. Evans (January 11, 1941 – April 29, 2022) was an American chemist who was the Abbott and James Lawrence professor of chemistry at Harvard University. He was a prominent figure in the field of organic chemistry and his research focus ...
, have been applied to many stereoselective transformations, including aldol reactions, alkylation reactions, and Diels-Alder reactions. The oxazolidinones are substituted at the 4 and 5 positions. Through steric hindrance, the substituents direct the direction of substitution of various groups. The auxiliary is subsequently removed e.g. through hydrolysis.
Preparation
Oxazolidinones can be prepared from amino acids or readily availableamino alcohols
In organic chemistry, alkanolamines are organic compounds that contain both hydroxyl () and amino (, , and ) functional groups on an alkane backbone. The term alkanolamine is a broad class term that is sometimes used as a subclassification.
Met ...
. A large number of oxazolidinones are commercially available, including the four below.
acyl chloride
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group . Their formula is usually written , where R is a side chain. They are reactive derivatives of carboxylic acids (). A specific example o ...
.

Alkylation reactions
Deprotonation at theα-carbon
In the nomenclature of organic chemistry, a locant is a term to indicate the position of a functional group or substituent within a molecule.
Numeric locants
The International Union of Pure and Applied Chemistry (IUPAC) recommends the use of ...
of an oxazolidinone imide
In organic chemistry, an imide is a functional group consisting of two acyl groups bound to nitrogen. The compounds are structurally related to acid anhydrides, although imides are more resistant to hydrolysis. In terms of commercial applications, ...
with a strong base such as lithium diisopropylamide selectively furnishes the (''Z'')- enolate, which can undergo stereoselective alkylation.
 Activated electrophiles, such as
Activated electrophiles, such as allyl
In organic chemistry, an allyl group is a substituent with the structural formula , where R is the rest of the molecule. It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, ...
ic or benzylic halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluor ...
s, are very good substrates.
Aldol reactions
Chiral oxazolidinones have been employed most widely in stereoselective aldol reactions. Soft enolization with theLewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
dibutylboron triflate and the base diisopropylethylamine
''N'',''N''-Diisopropylethylamine, or Hünig's base, is an organic compound and an amine. It is named after the German chemist Siegfried Hünig. It is used in organic chemistry as a base. It is commonly abbreviated as DIPEA, DIEA, or ''i''-Pr2N ...
gives the (''Z'')-enolate, which undergoes a diastereoselective aldol reaction with an aldehyde substrate. The transformation is particularly powerful because it establishes two contiguous stereocenters simultaneously.
 A model for the observed stereoselectivity can be found below. The ''syn''-stereorelationship between the methyl group and the new secondary alcohol results from a six-membered ring Zimmerman-Traxler transition state, wherein the enolate oxygen and the aldheyde oxygen both coordinate boron. The aldehyde is oriented such that the hydrogen is placed in a pseudo-axial orientation to minimize 1,3-diaxial interactions. The absolute stereochemistry of the two stereocenters is controlled by the chirality in the auxiliary. In the transition structure, the auxiliary carbonyl is oriented away from the enolate oxygen so as to minimize the net dipole of the molecule; one face of the enolate is blocked by the substituent on the chiral auxiliary.
A model for the observed stereoselectivity can be found below. The ''syn''-stereorelationship between the methyl group and the new secondary alcohol results from a six-membered ring Zimmerman-Traxler transition state, wherein the enolate oxygen and the aldheyde oxygen both coordinate boron. The aldehyde is oriented such that the hydrogen is placed in a pseudo-axial orientation to minimize 1,3-diaxial interactions. The absolute stereochemistry of the two stereocenters is controlled by the chirality in the auxiliary. In the transition structure, the auxiliary carbonyl is oriented away from the enolate oxygen so as to minimize the net dipole of the molecule; one face of the enolate is blocked by the substituent on the chiral auxiliary.

Removal
A variety of transformations have been developed to facilitate removal of the oxazolidinone auxiliary to generate different synthetically useful functional groups.
Camphorsultam
Camphorsultam
Camphorsultam, also known as bornanesultam, is a crystalline solid primarily used as a chiral auxiliary in the synthesis of other chemicals with a specific desired stereoselectivity. Camphorsultam is commercially available in both enantiomers of i ...
, or Oppolzer's sultam, is a classic chiral auxiliary.
oxazoline
Oxazoline is a five-membered heterocyclic organic compound with the formula . It is the parent of a family of compounds called oxazolines (emphasis on plural), which contain non-hydrogenic substituents on carbon and/or nitrogen. Oxazolines are the ...
ring asymmetrically. Comparing with oxazolidinone as the chiral auxiliary, camphorsultam had a significant (2''S'',3''R'')-selectivity.
 Camphorsultam also acts as a chiral auxiliary in Michael addition. Lithium base promoted stereoselective Michael addition of thiols to N-mcthacryloylcamphorsultam produced the corresponding addition products in high diastereoselectivity.
Camphorsultam also acts as a chiral auxiliary in Michael addition. Lithium base promoted stereoselective Michael addition of thiols to N-mcthacryloylcamphorsultam produced the corresponding addition products in high diastereoselectivity.
 Camphorsultam was used as a chiral auxiliary for the asymmetric Claisen rearrangement. In the presence of
Camphorsultam was used as a chiral auxiliary for the asymmetric Claisen rearrangement. In the presence of butylated hydroxytoluene
Butylated hydroxytoluene (BHT), also known as dibutylhydroxytoluene, is a lipophilic organic compound, chemically a derivative of phenol, that is useful for its antioxidant properties. BHT is widely used to prevent free radical-mediated oxidatio ...
(BHT) used as a radical scavenger, a toluene solution of the adduct between geraniol and camphorsultam was heated in a sealed tube at 140 °C, to provide mainly the (2''R'',3''S'')-isomer as the major rearrangement product in 72% yield, securing the two contiguous stereocenters including the quaternary carbon.

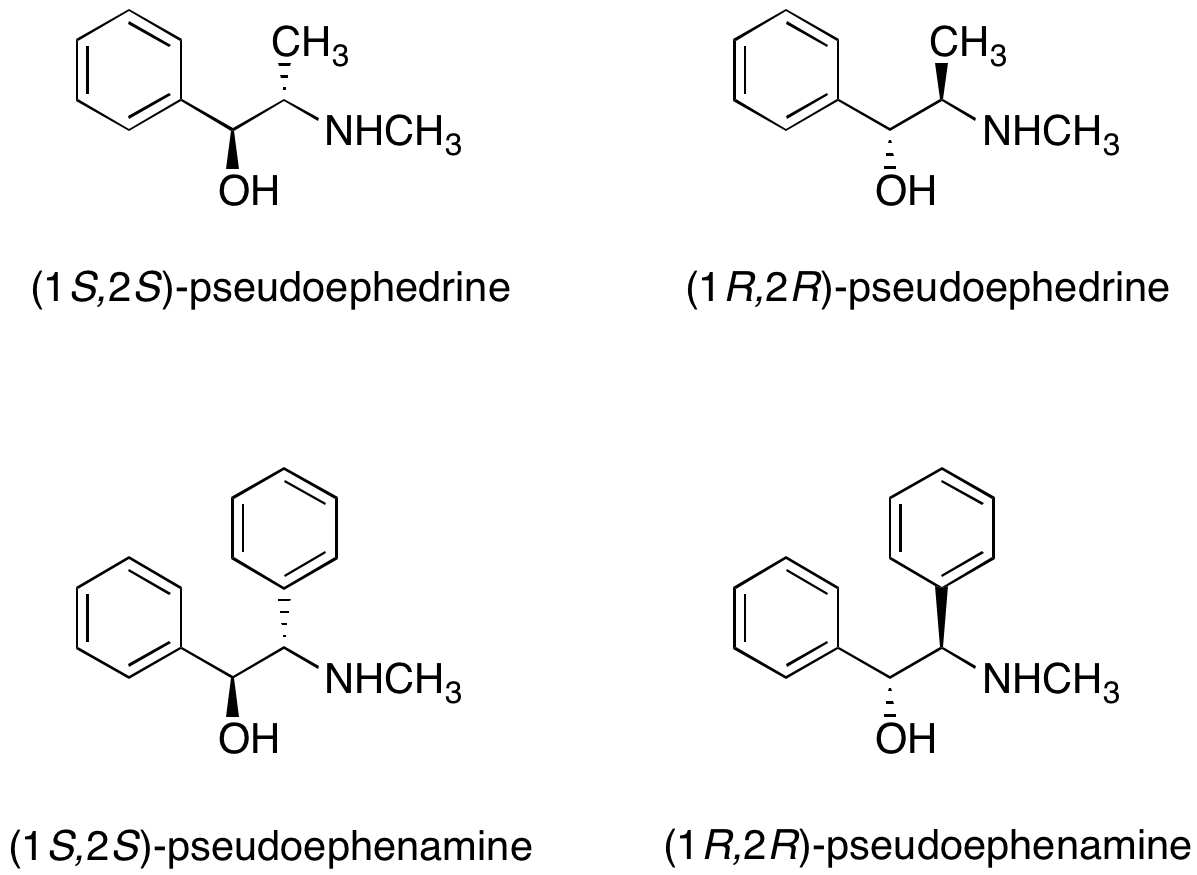
Pseudoephedrine and pseudoephenamine
Both (R,R)- and (S,S)-pseudoephedrine
Pseudoephedrine (PSE) is a sympathomimetic drug of the phenethylamine and amphetamine chemical classes. It may be used as a nasal/sinus decongestant, as a stimulant, or as a wakefulness-promoting agent in higher doses.
It was first characteri ...
can be used as chiral auxiliaries. Pseudoephedrine is reacted with a carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
, acid anhydride, or acyl chloride
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group . Their formula is usually written , where R is a side chain. They are reactive derivatives of carboxylic acids (). A specific example o ...
to give the corresponding amide.
The α-proton of the carbonyl compound is easily deprotonated by a non-nucleophilic base to give the enolate, which can further react. The configuration of the addition compound, such as with an alkyl halide, is directed by the methyl group. Thus, any addition product will be syn with the methyl and anti to the hydroxyl group
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy g ...
. The pseudoephedrine chiral auxiliary is subsequently removed by cleaving the amide bond with an appropriate nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
.
Preparation
Bothenantiomers
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical anti ...
of pseudoephedrine are commercially available. Racemic pseudoephedrine has many medical uses. Because pseudoephedrine can be used to illegally make methamphetamine
Methamphetamine (contracted from ) is a potent central nervous system (CNS) stimulant that is mainly used as a recreational drug and less commonly as a second-line treatment for attention deficit hyperactivity disorder and obesity. Methamph ...
, the purchase of pseudoephedrine for use in academic or industrial research is rather regulated. As an alternative, Myers et al. reported the utility of pseudoephenamine chiral auxiliaries in alkylation reactions. While pseudoephenamine is not readily available from commercial sources, it can be synthesized with relative ease from benzil and cannot be used to make amphetamine
Amphetamine (contracted from alpha- methylphenethylamine) is a strong central nervous system (CNS) stimulant that is used in the treatment of attention deficit hyperactivity disorder (ADHD), narcolepsy, and obesity. It is also commonly used ...
s.
 Pseudoephedrine amides are typically prepared by acylation with an acyl chloride or anhydride.
Pseudoephedrine amides are typically prepared by acylation with an acyl chloride or anhydride.

Alkylation
Pseudoephedrine amides undergo deprotonation by a strong base such as lithium diisopropylamide (LDA) to give the corresponding (''Z'')- enolates. Alkylation of these lithium enolates proceeds with high facial selectivity.
The diastereoselectivity is believed to result from a configuration wherein one face of the lithium enolate is blocked by the secondary lithium alkoxide and the solvent molecules associated with that lithium cation. In accordance with this proposal, it has been observed that the diastereoselctivity of the alkylation step is highly dependent on the amount of lithium chloride present and on the solvent, tetrahydrofuran (THF). Typically, 4 to 6 equivalents of lithium chloride are sufficient to saturate a solution of enolate in THF at the reaction molarity.

One primary advantage of asymmetric alkylation with pseudoephedrine amides is that the amide enolates are typically nucleophilic enough to react with primary and even secondary halides at temperatures ranging from –78 °C to 0 °C. Construction of quaternary carbon centers by alkylation of α-branched amide enolates is also possible, though the addition of
DMPU
''N'',''N''′-Dimethylpropyleneurea (DMPU) is a cyclic urea sometimes used as a polar, aprotic organic solvent. In 1985, Dieter Seebach showed that it is possible to replace the suspected carcinogen hexamethylphosphoramide
Hexamethylphosphorami ...
is necessary for less reactive electrophiles.
Removal
Conditions have been developed for the transformation of pseudoephedrine amides into enantiomerically enrichedcarboxylic acids
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
, alcohols
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl () functional group bound to a saturated carbon atom. The term ''alcohol'' originally referred to the primary alcohol ethanol (ethyl alcohol), which is ...
, aldehydes, and ketones
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bon ...
- after cleavage, the auxiliary can be recovered and reused.

''tert''-Butanesulfinamide
This specificsulfinamide
Sulfinamide is a functional group in organosulfur chemistry with the structural formula RS(O)NR'2 (where R and R' are organic substituents). This functionality is composed of a sulfur-carbon (S–C) and sulfur-nitrogen (S–N) single bonds, as we ...
chiral auxiliary was initially developed by Jonathan A. Ellman, and its use has been explored extensively by his group. Thus, it is often referred to as Ellman's auxiliary or Ellman's sulfinamide.

Preparation
Either enantiomer of ''tert''-butanesulfinamide can be reached from ''tert''-butyl disulfide in two steps: a catalytic asymmetric oxidation reaction gives the disulfide monooxidation product in high yield and enantiomeric excess. Treatment of this compound with lithium amide in ammonia affords optically pure inverted product.
Condensation of ''tert''-butanesulfinamide with an aldehyde or ketone proceeds in high yield and affords only the (''E'')-isomer of the corresponding ''N''-sulfinyl imines.

Synthesis of chiral amines
Addition of a Grignard reagent to a ''tert''-butanesulfinyl aldimine or ketimine results in asymmetric addition to give the branched sulfinamide. The observed stereoselectivity can be rationalized by a six-membered ring transition structure, wherein both oxygen and nitrogen of the sulfinyl imine coordinate magnesium.
Removal
The auxiliary can be removed from the desired amine by treatment with hydrochloric acid in protic solvents.
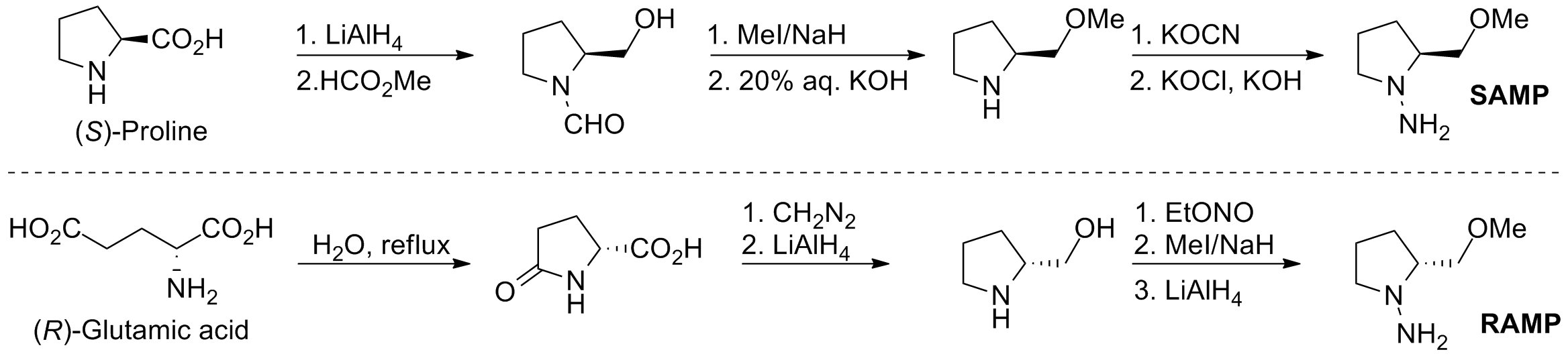
SAMP/RAMP
Alkylation reactions of chiral (''S'')-1-amino-2-methoxymethylpyrrolidine (SAMP
Samp is an African food consisting of dried corn kernels that have been pounded and chopped until broken, but not as finely ground as mealie-meal or mielie rice. The coating around the kernel loosens and is removed during the pounding and stamp ...
) and (''R'')-1-amino-2-methoxymethylpyrrolidine (RAMP
An inclined plane, also known as a ramp, is a flat supporting surface tilted at an angle from the vertical direction, with one end higher than the other, used as an aid for raising or lowering a load. The inclined plane is one of the six clas ...
) hydrazones were developed by Dieter Enders and E.J. Corey.
Preparation
SAMP can be prepared in six steps from (''S'')-proline, and RAMP can be prepared in six steps from (''R'')-glutamic acid.
Alkylation reactions
Condensation of SAMP or RAMP with an aldehyde or ketone affords the (''E'')-hydrazine. Deprotonation with lithium diisopropylamide and addition of an alkyl halide affords the alkylated product. The auxiliary can be removed by ozonolysis or hydrolysis.
Chiral auxiliaries in industry
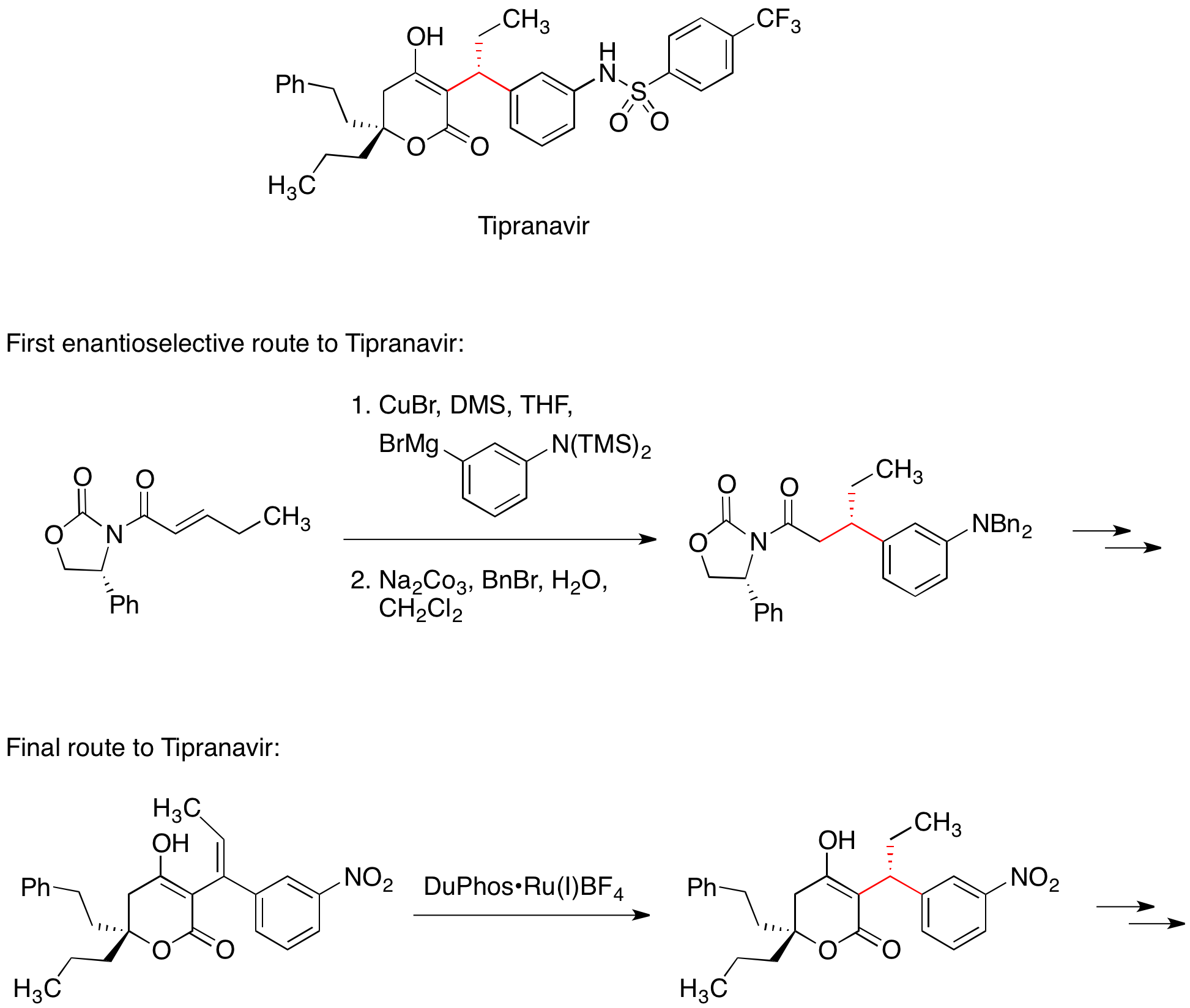
Chiral auxiliaries are generally reliable and versatile, enabling the synthesis of a large number of enantiomerically pure compounds in a time-efficient manner. Consequently, chiral auxiliaries are often the method of choice in the early phases of drug development.Tipranavir
The HIV protease inhibitorTipranavir
Tipranavir (TPV), or tipranavir disodium, is a nonpeptidic protease inhibitor (PI) manufactured by Boehringer Ingelheim under the trade name Aptivus . It is administered with ritonavir in combination therapy to treat HIV infection.
Tipranavir ...
is marketed for the treatment of AIDS. The first enantioselective medicinal chemistry route to Tipranavir included the conjugate addition of an organocuprate reagent to a chiral Michael acceptor. The chiral oxazolidinone in the Michael acceptor controlled the stereochemistry of one of two stereocenters in the molecule. The final, commercial route to Tipranavir does not feature a chiral auxiliary; instead, this stereocenter is set by an asymmetric hydrogenation reaction.

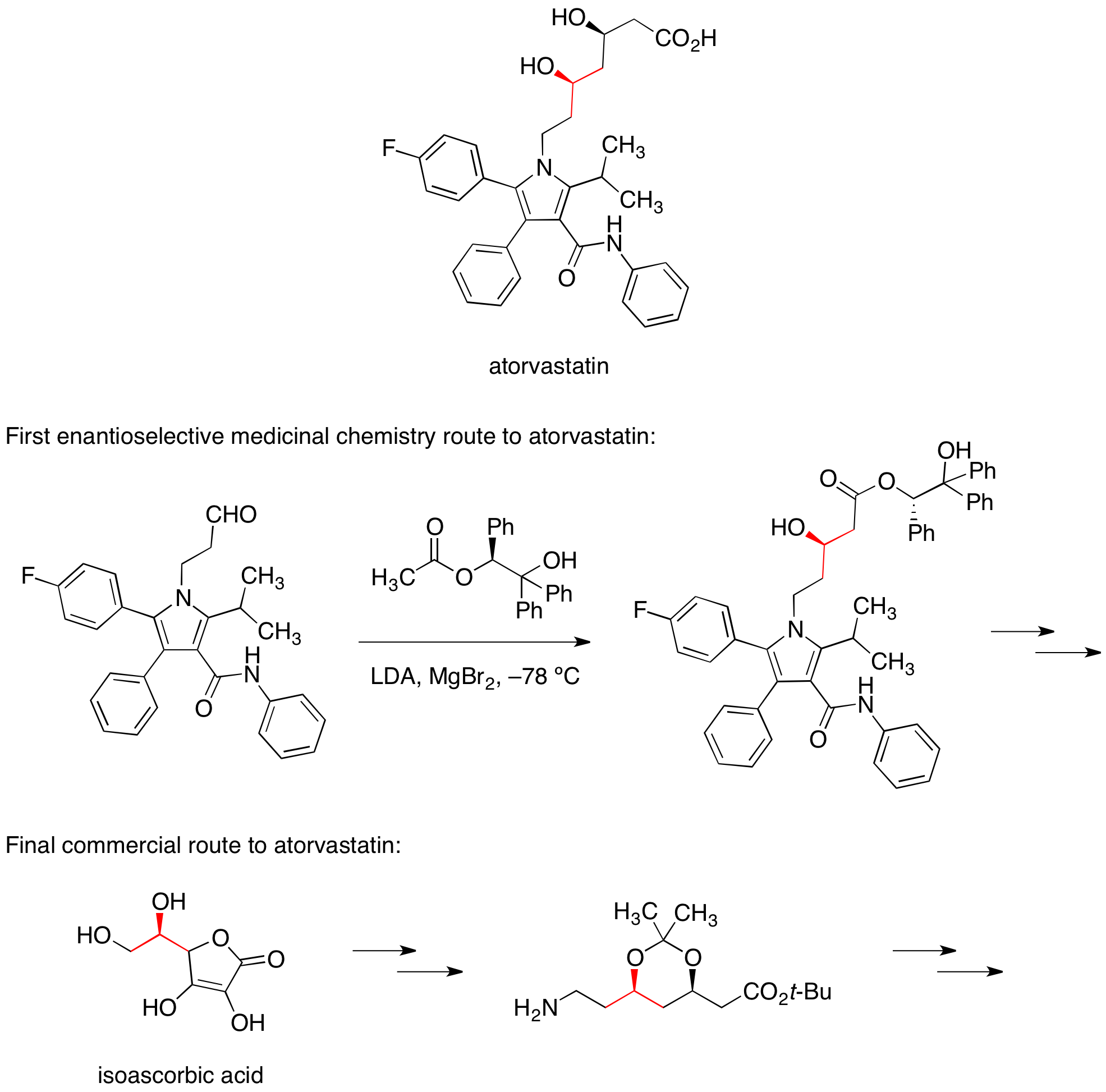
Atorvastatin
The calcium salt of atorvastatin is marketed under the trade name Lipitor for the lowering of blood cholesterol. The first enantioselective medicinal chemistry route to atorvastatin relied on a diastereoselective aldol reaction with a chiral ester to set one of the two alcohol stereocenters. In the commercial route to atorvastatin, this stereocenter is carried forward from the readily availablefood additive
Food additives are substances added to food to preserve flavor or enhance taste, appearance, or other sensory qualities. Some additives have been used for centuries as part of an effort to preserve food, for example vinegar (pickling), salt (salt ...
isoascorbic acid
Erythorbic acid (isoascorbic acid, D-araboascorbic acid) is a stereoisomer of ascorbic acid (vitamin C). It is synthesized by a reaction between methyl 2-keto-D-gluconate and sodium methoxide. It can also be synthesized from sucrose or by strain ...
.

See also
* Example of use of trans-2-phenyl-1-cyclohexanol as chiral auxiliary:Ojima lactam Ojima may refer to:
*Ojima (surname), a Japanese surname
*Ojima, Gunma, a town merged into the city of Ōta, Gunma Prefecture, Japan
*Ojima Station, a railway station in Kōtō, Tokyo, Japan
*Higashi-ojima Station, a railway station in Kōtō, Toky ...
* Valine as a Chiral auxiliary in the Schöllkopf method The Schöllkopf method or Schöllkopf Bis-Lactim Amino Acid Synthesis is a method in organic chemistry for the asymmetric synthesis of chiral amino acids.Jan Bülle, Aloys Hüttermann : ''Das Basiswissen der organischen Chemie: Die wichtigsten organ ...
References
{{Reflist Stereochemistry