Molecule 1 on:
[Wikipedia]
[Google]
[Amazon]

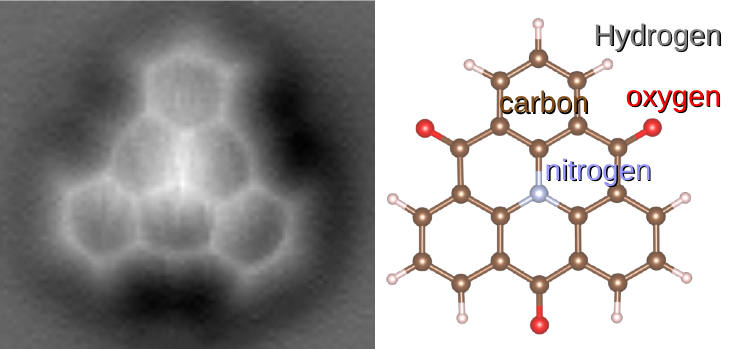 A molecule is a group of two or more
A molecule is a group of two or more
 ),
),  ),
),  ), and
), and  )) and "forces" of attraction and repulsion allowing the elements to interact.
A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by
)) and "forces" of attraction and repulsion allowing the elements to interact.
A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by 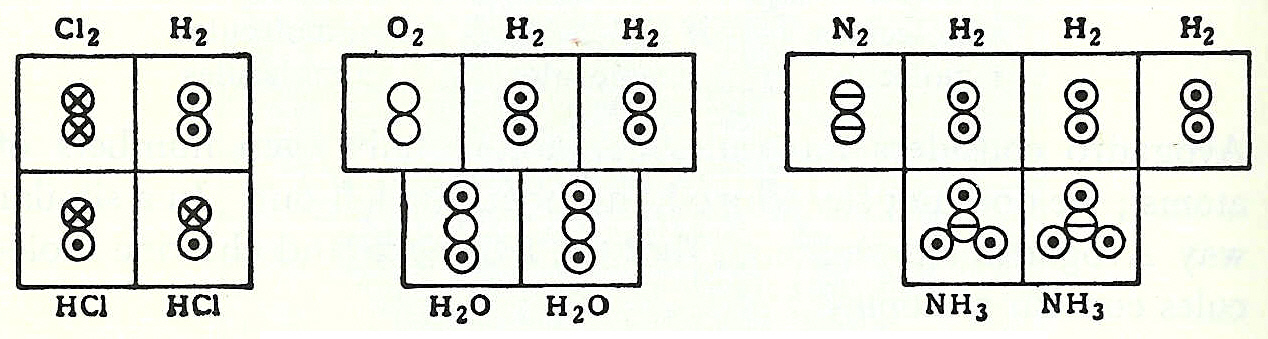 In 1917, an unknown American undergraduate chemical engineer named Linus Pauling was learning the Dalton hook-and-eye bonding method, which was the mainstream description of bonds between atoms at the time. Pauling, however, wasn't satisfied with this method and looked to the newly emerging field of quantum physics for a new method. In 1926, French physicist Jean Perrin received the Nobel Prize in physics for proving, conclusively, the existence of molecules. He did this by calculating
In 1917, an unknown American undergraduate chemical engineer named Linus Pauling was learning the Dalton hook-and-eye bonding method, which was the mainstream description of bonds between atoms at the time. Pauling, however, wasn't satisfied with this method and looked to the newly emerging field of quantum physics for a new method. In 1926, French physicist Jean Perrin received the Nobel Prize in physics for proving, conclusively, the existence of molecules. He did this by calculating 
 A covalent bond is a chemical bond that involves the sharing of
A covalent bond is a chemical bond that involves the sharing of
 Ionic bonding is a type of chemical bond that involves the
Ionic bonding is a type of chemical bond that involves the
 For molecules with a complicated 3-dimensional structure, especially involving atoms bonded to four different substituents, a simple molecular formula or even semi-structural chemical formula may not be enough to completely specify the molecule. In this case, a graphical type of formula called a structural formula may be needed. Structural formulas may in turn be represented with a one-dimensional chemical name, but such chemical nomenclature requires many words and terms which are not part of chemical formulas.
For molecules with a complicated 3-dimensional structure, especially involving atoms bonded to four different substituents, a simple molecular formula or even semi-structural chemical formula may not be enough to completely specify the molecule. In this case, a graphical type of formula called a structural formula may be needed. Structural formulas may in turn be represented with a one-dimensional chemical name, but such chemical nomenclature requires many words and terms which are not part of chemical formulas.
 Molecules have fixed equilibrium geometries—bond lengths and angles— about which they continuously oscillate through vibrational and rotational motions. A pure substance is composed of molecules with the same average geometrical structure. The chemical formula and the structure of a molecule are the two important factors that determine its properties, particularly its reactivity.
Molecules have fixed equilibrium geometries—bond lengths and angles— about which they continuously oscillate through vibrational and rotational motions. A pure substance is composed of molecules with the same average geometrical structure. The chemical formula and the structure of a molecule are the two important factors that determine its properties, particularly its reactivity.
 Molecular spectroscopy deals with the response (
Molecular spectroscopy deals with the response (
Molecule of the MonthSchool of Chemistry, University of Bristol
{{Authority control Chemistry Matter

 A molecule is a group of two or more
A molecule is a group of two or more atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, ...
s held together by attractive forces known as chemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of ...
s; depending on context, the term may or may not include ions
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
which satisfy this criterion. In quantum physics, organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, ...
, and biochemistry
Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology and ...
, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ion
A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zero. The term molecule may or may no ...
s.
A molecule may be homonuclear
Homonuclear molecules, or homonuclear species, are molecules composed of only one element. Homonuclear molecules may consist of various numbers of atoms. The size of the molecule an element can form depends on the element's properties, and some el ...
, that is, it consists of atoms of one chemical element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler sub ...
, e.g. two atoms in the oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
molecule (O2); or it may be heteronuclear
A heteronuclear molecule is a molecule composed of atoms of more than one chemical element. For example, a molecule of water (H2O) is heteronuclear because it has atoms of two different elements, hydrogen (H) and oxygen (O).
Similarly, a heteronu ...
, a chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
composed of more than one element, e.g. water
Water (chemical formula ) is an Inorganic compound, inorganic, transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living ...
(two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle
In the physical sciences, a particle (or corpuscule in older texts) is a small localized object which can be described by several physical or chemical properties, such as volume, density, or mass.
They vary greatly in size or quantity, from ...
regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases
The noble gases (historically also the inert gases; sometimes referred to as aerogens) make up a class of chemical elements with similar properties; under standard conditions, they are all odorless, colorless, monatomic gases with very low ch ...
are individual atoms. Atoms and complexes connected by non-covalent interactions
In chemistry, a non-covalent interaction differs from a covalent bond in that it does not involve the sharing of electrons, but rather involves more dispersed variations of electromagnetic interactions between molecules or within a molecule. The ...
, such as hydrogen bonds or ionic bond
Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with sharply different electronegativities, and is the primary interaction occurring in ionic compounds ...
s, are typically not considered single molecules.
Concepts similar to molecules have been discussed since ancient times, but modern investigation into the nature of molecules and their bonds began in the 17th century. Refined over time by scientists such as Robert Boyle
Robert Boyle (; 25 January 1627 – 31 December 1691) was an Anglo-Irish natural philosopher, chemist, physicist, alchemist and inventor. Boyle is largely regarded today as the first modern chemist, and therefore one of the founders of ...
, Amedeo Avogadro
Lorenzo Romano Amedeo Carlo Avogadro, Count of Quaregna and Cerreto (, also , ; 9 August 17769 July 1856) was an Italian scientist, most noted for his contribution to molecular theory now known as Avogadro's law, which states that equal volume ...
, Jean Perrin, and Linus Pauling, the study of molecules is today known as molecular physics
Molecular physics is the study of the physical properties of molecules and molecular dynamics. The field overlaps significantly with physical chemistry, chemical physics, and quantum chemistry. It is often considered as a sub-field of atomic, m ...
or molecular chemistry.
Etymology
According to Merriam-Webster and the Online Etymology Dictionary, the word "molecule" derives from theLatin
Latin (, or , ) is a classical language belonging to the Italic branch of the Indo-European languages. Latin was originally a dialect spoken in the lower Tiber area (then known as Latium) around present-day Rome, but through the power of the ...
"moles Moles can refer to:
* Moles de Xert, a mountain range in the Baix Maestrat comarca, Valencian Community, Spain
* The Moles (Australian band)
*The Moles, alter ego of Scottish band Simon Dupree and the Big Sound
People
*Abraham Moles, French engin ...
" or small unit of mass. The word is derived from French ' (1678), from New Latin
New Latin (also called Neo-Latin or Modern Latin) is the revival of Literary Latin used in original, scholarly, and scientific works since about 1500. Modern scholarly and technical nomenclature, such as in zoological and botanical taxonomy ...
', diminutive of Latin ' "mass, barrier". The word, which until the late 18th century was used only in Latin form, became popular after being used in works of philosophy by Descartes.History
The definition of the molecule has evolved as knowledge of the structure of molecules has increased. Earlier definitions were less precise, defining molecules as the smallestparticles
In the physical sciences, a particle (or corpuscule in older texts) is a small localized object which can be described by several physical or chemical properties, such as volume, density, or mass.
They vary greatly in size or quantity, from s ...
of pure chemical substance
A chemical substance is a form of matter having constant chemical composition and characteristic properties. Some references add that chemical substance cannot be separated into its constituent elements by physical separation methods, i.e., w ...
s that still retain their composition
Composition or Compositions may refer to:
Arts and literature
*Composition (dance), practice and teaching of choreography
*Composition (language), in literature and rhetoric, producing a work in spoken tradition and written discourse, to include v ...
and chemical properties. This definition often breaks down since many substances in ordinary experience, such as rocks
In geology, rock (or stone) is any naturally occurring solid mass or aggregate of minerals or mineraloid matter. It is categorized by the minerals included, its chemical composition, and the way in which it is formed. Rocks form the Earth's ...
, salts
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively c ...
, and metal
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typicall ...
s, are composed of large crystalline networks of chemically bonded atoms or ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
s, but are not made of discrete molecules.
The modern concept of molecules can be traced back towards pre-scientific and Greek philosophers such as Leucippus
Leucippus (; el, Λεύκιππος, ''Leúkippos''; fl. 5th century BCE) is a pre-Socratic Greek philosopher who has been credited as the first philosopher to develop a theory of atomism.
Leucippus' reputation, even in antiquity, was obscured ...
and Democritus
Democritus (; el, Δημόκριτος, ''Dēmókritos'', meaning "chosen of the people"; – ) was an Ancient Greek pre-Socratic philosopher from Abdera, primarily remembered today for his formulation of an atomic theory of the universe. No ...
who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles
Empedocles (; grc-gre, Ἐμπεδοκλῆς; , 444–443 BC) was a Greek pre-Socratic philosopher and a native citizen of Akragas, a Greek city in Sicily. Empedocles' philosophy is best known for originating the cosmogonic theory of the ...
imagined fundamental elements (fire
Fire is the rapid oxidation of a material (the fuel) in the exothermic chemical process of combustion, releasing heat, light, and various reaction products.
At a certain point in the combustion reaction, called the ignition point, flames a ...
(earth
Earth is the third planet from the Sun and the only astronomical object known to harbor life. While large volumes of water can be found throughout the Solar System, only Earth sustains liquid surface water. About 71% of Earth's surfa ...
(air
The atmosphere of Earth is the layer of gases, known collectively as air, retained by Earth's gravity that surrounds the planet and forms its planetary atmosphere. The atmosphere of Earth protects life on Earth by creating pressure allowing f ...
(water
Water (chemical formula ) is an Inorganic compound, inorganic, transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living ...
(Aristotle
Aristotle (; grc-gre, Ἀριστοτέλης ''Aristotélēs'', ; 384–322 BC) was a Greek philosopher and polymath during the Classical period in Ancient Greece. Taught by Plato, he was the founder of the Peripatetic school of ph ...
and passed to medieval and renaissance Europe.
In a more concrete manner, however, the concept of aggregates or units of bonded atoms, i.e. "molecules", traces its origins to Robert Boyle
Robert Boyle (; 25 January 1627 – 31 December 1691) was an Anglo-Irish natural philosopher, chemist, physicist, alchemist and inventor. Boyle is largely regarded today as the first modern chemist, and therefore one of the founders of ...
's 1661 hypothesis, in his famous treatise ''The Sceptical Chymist
''The Sceptical Chymist: or Chymico-Physical Doubts & Paradoxes'' is the title of a book by Robert Boyle, published in London in 1661. In the form of a dialogue, the ''Sceptical Chymist'' presented Boyle's hypothesis that matter consisted of corp ...
'', that matter is composed of ''clusters of particles'' and that chemical change results from the rearrangement of the clusters. Boyle argued that matter's basic elements consisted of various sorts and sizes of particles, called "corpuscles", which were capable of arranging themselves into groups. In 1789, William Higgins published views on what he called combinations of "ultimate" particles, which foreshadowed the concept of valency bonds
In chemistry, valence bond (VB) theory is one of the two basic theories, along with molecular orbital theory, molecular orbital (MO) theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on ho ...
. If, for example, according to Higgins, the force between the ultimate particle of oxygen and the ultimate particle of nitrogen were 6, then the strength of the force would be divided accordingly, and similarly for the other combinations of ultimate particles.
Amedeo Avogadro
Lorenzo Romano Amedeo Carlo Avogadro, Count of Quaregna and Cerreto (, also , ; 9 August 17769 July 1856) was an Italian scientist, most noted for his contribution to molecular theory now known as Avogadro's law, which states that equal volume ...
created the word "molecule". His 1811 paper "Essay on Determining the Relative Masses of the Elementary Molecules of Bodies", he essentially states, i.e. according to Partington
Partington is a town and civil parish in the Metropolitan Borough of Trafford, Greater Manchester, England, south-west of Manchester city centre. Within the boundaries of the historic county of Cheshire, it lies on the southern bank of the M ...
's ''A Short History of Chemistry'', that:In coordination with these concepts, in 1833 the French chemist Marc Antoine Auguste Gaudin presented a clear account of Avogadro's hypothesis, regarding atomic weights, by making use of "volume diagrams", which clearly show both semi-correct molecular geometries, such as a linear water molecule, and correct molecular formulas, such as H2O:
 In 1917, an unknown American undergraduate chemical engineer named Linus Pauling was learning the Dalton hook-and-eye bonding method, which was the mainstream description of bonds between atoms at the time. Pauling, however, wasn't satisfied with this method and looked to the newly emerging field of quantum physics for a new method. In 1926, French physicist Jean Perrin received the Nobel Prize in physics for proving, conclusively, the existence of molecules. He did this by calculating
In 1917, an unknown American undergraduate chemical engineer named Linus Pauling was learning the Dalton hook-and-eye bonding method, which was the mainstream description of bonds between atoms at the time. Pauling, however, wasn't satisfied with this method and looked to the newly emerging field of quantum physics for a new method. In 1926, French physicist Jean Perrin received the Nobel Prize in physics for proving, conclusively, the existence of molecules. He did this by calculating Avogadro's number
The Avogadro constant, commonly denoted or , is the proportionality factor that relates the number of constituent particles (usually molecules, atoms or ions) in a sample with the amount of substance in that sample. It is an SI defining co ...
using three different methods, all involving liquid phase systems. First, he used a gamboge
Gamboge ( , ) is a partially transparent deep saffron to mustard yellow pigment.Other forms and spellings are: cambodia, cambogium, camboge, cambugium, gambaugium, gambogia, gambozia, gamboidea, gambogium, gumbouge, gambouge, gamboge, gambooge, g ...
soap-like emulsion, second by doing experimental work on Brownian motion
Brownian motion, or pedesis (from grc, πήδησις "leaping"), is the random motion of particles suspended in a medium (a liquid or a gas).
This pattern of motion typically consists of random fluctuations in a particle's position insi ...
, and third by confirming Einstein's theory of particle rotation in the liquid phase.
In 1927, the physicists Fritz London
Fritz Wolfgang London (March 7, 1900 – March 30, 1954) was a German physicist and professor at Duke University. His fundamental contributions to the theories of chemical bonding and of intermolecular forces ( London dispersion forces) are today ...
and Walter Heitler
Walter Heinrich Heitler (; 2 January 1904 – 15 November 1981) was a German physicist who made contributions to quantum electrodynamics and quantum field theory. He brought chemistry under quantum mechanics through his theory of valence bond ...
applied the new quantum mechanics to the deal with the saturable, nondynamic forces of attraction and repulsion, i.e., exchange forces, of the hydrogen molecule. Their valence bond treatment of this problem, in their joint paper, was a landmark in that it brought chemistry under quantum mechanics. Their work was an influence on Pauling, who had just received his doctorate and visited Heitler and London in Zürich on a Guggenheim Fellowship.
Subsequently, in 1931, building on the work of Heitler and London and on theories found in Lewis' famous article, Pauling published his ground-breaking article "The Nature of the Chemical Bond" in which he used quantum mechanics
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistr ...
to calculate properties and structures of molecules, such as angles between bonds and rotation about bonds. On these concepts, Pauling developed hybridization theory
In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new ''hybrid orbitals'' (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to f ...
to account for bonds in molecules such as CH4, in which four sp³ hybridised orbitals are overlapped by hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
's ''1s'' orbital, yielding four sigma (σ) bonds. The four bonds are of the same length and strength, which yields a molecular structure as shown below:
Molecular science
The science of molecules is called ''molecular chemistry'' or ''molecular physics
Molecular physics is the study of the physical properties of molecules and molecular dynamics. The field overlaps significantly with physical chemistry, chemical physics, and quantum chemistry. It is often considered as a sub-field of atomic, m ...
'', depending on whether the focus is on chemistry or physics. Molecular chemistry deals with the laws governing the interaction between molecules that results in the formation and breakage of chemical bonds, while molecular physics deals with the laws governing their structure and properties. In practice, however, this distinction is vague. In molecular sciences, a molecule consists of a stable system (bound state
Bound or bounds may refer to:
Mathematics
* Bound variable
* Upper and lower bounds, observed limits of mathematical functions
Physics
* Bound state, a particle that has a tendency to remain localized in one or more regions of space
Geography
* ...
) composed of two or more atoms. Polyatomic ion
A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zero. The term molecule may or may no ...
s may sometimes be usefully thought of as electrically charged molecules. The term ''unstable molecule'' is used for very reactive
Reactive may refer to:
*Generally, capable of having a reaction (disambiguation)
*An adjective abbreviation denoting a bowling ball coverstock made of reactive resin
*Reactivity (chemistry)
*Reactive mind
*Reactive programming
See also
*Reactanc ...
species, i.e., short-lived assemblies (resonances
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied periodic force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts. When an oscillat ...
) of electrons and nuclei, such as radicals, molecular ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
s, Rydberg molecules, transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked ...
s, van der Waals complexes, or systems of colliding atoms as in Bose–Einstein condensate
In condensed matter physics, a Bose–Einstein condensate (BEC) is a state of matter that is typically formed when a gas of bosons at very low densities is cooled to temperatures very close to absolute zero (−273.15 °C or −459.6 ...
.
Prevalence
Molecules as components of matter are common. They also make up most of the oceans and atmosphere. Most organic substances are molecules. The substances of life are molecules, e.g. proteins, the amino acids of which they are composed, the nucleic acids (DNA and RNA), sugars, carbohydrates, fats, and vitamins. The nutrient minerals are generally ionic compounds, thus they are not molecules, e.g. iron sulfate. However, the majority of familiar solid substances on Earth are made partly or completely of crystals or ionic compounds, which are not made of molecules. These include all of the minerals that make up the substance of the Earth, sand, clay, pebbles, rocks, boulders, bedrock, the molten interior, and the core of the Earth. All of these contain many chemical bonds, but are ''not'' made of identifiable molecules. No typical molecule can be defined for salts nor for covalent crystals, although these are often composed of repeatingunit cell
In geometry, biology, mineralogy and solid state physics, a unit cell is a repeating unit formed by the vectors spanning the points of a lattice. Despite its suggestive name, the unit cell (unlike a unit vector, for example) does not necessaril ...
s that extend either in a plane
Plane(s) most often refers to:
* Aero- or airplane, a powered, fixed-wing aircraft
* Plane (geometry), a flat, 2-dimensional surface
Plane or planes may also refer to:
Biology
* Plane (tree) or ''Platanus'', wetland native plant
* ''Planes' ...
, e.g. graphene
Graphene () is an allotrope of carbon consisting of a single layer of atoms arranged in a hexagonal lattice nanostructure.
; or three-dimensionally e.g. diamond
Diamond is a solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Another solid form of carbon known as graphite is the chemically stable form of carbon at room temperature and pressure, ...
, quartz
Quartz is a hard, crystalline mineral composed of silica ( silicon dioxide). The atoms are linked in a continuous framework of SiO4 silicon-oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall chemical ...
, sodium chloride. The theme of repeated unit-cellular-structure also holds for most metals which are condensed phases with metallic bond
Metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons (in the form of an electron cloud of delocalized electrons) and positively charged metal ions. It may be des ...
ing. Thus solid metals are not made of molecules. In glass
Glass is a non-crystalline, often transparent, amorphous solid that has widespread practical, technological, and decorative use in, for example, window panes, tableware, and optics. Glass is most often formed by rapid cooling ( quenching ...
es, which are solids that exist in a vitreous disordered state, the atoms are held together by chemical bonds with no presence of any definable molecule, nor any of the regularity of repeating unit-cellular-structure that characterizes salts, covalent crystals, and metals.
Bonding
Molecules are generally held together bycovalent bonding
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
. Several non-metallic elements exist only as molecules in the environment either in compounds or as homonuclear molecules, not as free atoms: for example, hydrogen.
While some people say a metallic crystal can be considered a single giant molecule held together by metallic bonding
Metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons (in the form of an electron cloud of delocalized electrons) and positively charged metal ions. It may be des ...
, others point out that metals behave very differently than molecules.
Covalent
electron pair
In chemistry, an electron pair or Lewis pair consists of two electrons that occupy the same molecular orbital but have opposite spins. Gilbert N. Lewis introduced the concepts of both the electron pair and the covalent bond in a landmark paper he ...
s between atoms. These electron pairs are termed ''shared pairs'' or ''bonding pairs'', and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is termed ''covalent bonding''.
Ionic
 Ionic bonding is a type of chemical bond that involves the
Ionic bonding is a type of chemical bond that involves the electrostatic
Electrostatics is a branch of physics that studies electric charges at rest ( static electricity).
Since classical times, it has been known that some materials, such as amber, attract lightweight particles after rubbing. The Greek word for amb ...
attraction between oppositely charged ions, and is the primary interaction occurring in ionic compounds. The ions are atoms that have lost one or more electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no ...
s (termed cations) and atoms that have gained one or more electrons (termed anion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
s). This transfer of electrons is termed ''electrovalence'' in contrast to covalence. In the simplest case, the cation is a metal
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typicall ...
atom and the anion is a nonmetal atom, but these ions can be of a more complicated nature, e.g. molecular ions like NH4+ or SO42−. At normal temperatures and pressures, ionic bonding mostly creates solids (or occasionally liquids) without separate identifiable molecules, but the vaporization/sublimation of such materials does produce separate molecules where electrons are still transferred fully enough for the bonds to be considered ionic rather than covalent.
Molecular size
Most molecules are far too small to be seen with the naked eye, although molecules of manypolymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
s can reach macroscopic
The macroscopic scale is the length scale on which objects or phenomena are large enough to be visible with the naked eye, without magnifying optical instruments. It is the opposite of microscopic.
Overview
When applied to physical phenomena a ...
sizes, including biopolymers such as DNA. Molecules commonly used as building blocks for organic synthesis have a dimension of a few angstrom
The angstromEntry "angstrom" in the Oxford online dictionary. Retrieved on 2019-03-02 from https://en.oxforddictionaries.com/definition/angstrom.Entry "angstrom" in the Merriam-Webster online dictionary. Retrieved on 2019-03-02 from https://www.m ...
s (Å) to several dozen Å, or around one billionth of a meter. Single molecules cannot usually be observed by light
Light or visible light is electromagnetic radiation that can be perceived by the human eye. Visible light is usually defined as having wavelengths in the range of 400–700 nanometres (nm), corresponding to frequencies of 750–420 te ...
(as noted above), but small molecules and even the outlines of individual atoms may be traced in some circumstances by use of an atomic force microscope
Atomic force microscopy (AFM) or scanning force microscopy (SFM) is a very-high-resolution type of scanning probe microscopy (SPM), with demonstrated resolution on the order of fractions of a nanometer, more than 1000 times better than the op ...
. Some of the largest molecules are macromolecules or supermolecule
The term supermolecule (or supramolecule) was introduced by Karl Lothar Wolf ''et al.'' (''Übermoleküle'') in 1937 to describe hydrogen-bonded acetic acid dimers. The study of non-covalent association of complexes of molecules has since develope ...
s.
The smallest molecule is the diatomic hydrogen (H2), with a bond length of 0.74 Å.
Effective molecular radius is the size a molecule displays in solution.
The table of permselectivity for different substances This is a table of permselectivity for different substances in the glomerulus of the kidney
The kidneys are two reddish-brown bean-shaped organs found in vertebrates. They are located on the left and right in the retroperitoneal space, and ...
contains examples.
Molecular formulas
Chemical formula types
Thechemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbol ...
for a molecule uses one line of chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, and ''plus'' (+) and ''minus'' (−) signs. These are limited to one typographic line of symbols, which may include subscripts and superscripts.
A compound's empirical formula
In chemistry, the empirical formula of a chemical compound is the simplest whole number ratio of atoms present in a compound. A simple example of this concept is that the empirical formula of sulfur monoxide, or SO, would simply be SO, as is the ...
is a very simple type of chemical formula. It is the simplest integer
An integer is the number zero (), a positive natural number (, , , etc.) or a negative integer with a minus sign ( −1, −2, −3, etc.). The negative numbers are the additive inverses of the corresponding positive numbers. In the languag ...
ratio
In mathematics, a ratio shows how many times one number contains another. For example, if there are eight oranges and six lemons in a bowl of fruit, then the ratio of oranges to lemons is eight to six (that is, 8:6, which is equivalent to the ...
of the chemical elements that constitute it. For example, water is always composed of a 2:1 ratio of hydrogen to oxygen atoms, and ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a ...
(ethyl alcohol) is always composed of carbon, hydrogen, and oxygen in a 2:6:1 ratio. However, this does not determine the kind of molecule uniquely – dimethyl ether
Dimethyl ether (DME; also known as methoxymethane) is the organic compound with the formula CH3OCH3,
(sometimes ambiguously simplified to C2H6O as it is an isomer of ethanol). The simplest ether, it is a colorless gas that is a useful precursor ...
has the same ratios as ethanol, for instance. Molecules with the same atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, ...
s in different arrangements are called isomers. Also carbohydrates, for example, have the same ratio (carbon:hydrogen:oxygen= 1:2:1) (and thus the same empirical formula) but different total numbers of atoms in the molecule.
The molecular formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
reflects the exact number of atoms that compose the molecule and so characterizes different molecules. However different isomers can have the same atomic composition while being different molecules.
The empirical formula is often the same as the molecular formula but not always. For example, the molecule acetylene has molecular formula C2H2, but the simplest integer ratio of elements is CH.
The molecular mass
The molecular mass (''m'') is the mass of a given molecule: it is measured in daltons (Da or u). Different molecules of the same compound may have different molecular masses because they contain different isotopes of an element. The related quanti ...
can be calculated from the chemical formula and is expressed in conventional atomic mass unit
The dalton or unified atomic mass unit (symbols: Da or u) is a non-SI unit of mass widely used in physics and chemistry. It is defined as of the mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state and at ...
s equal to 1/12 of the mass of a neutral carbon-12 (12 C isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numb ...
) atom. For network solids, the term formula unit
In chemistry, a formula unit is the empirical formula of any ionic or covalent network solid compound used as an independent entity for stoichiometric calculations. It is the lowest whole number ratio of ions represented in an ionic compound. E ...
is used in stoichiometric
Stoichiometry refers to the relationship between the quantities of reactants and products before, during, and following chemical reactions.
Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equ ...
calculations.
Structural formula
 For molecules with a complicated 3-dimensional structure, especially involving atoms bonded to four different substituents, a simple molecular formula or even semi-structural chemical formula may not be enough to completely specify the molecule. In this case, a graphical type of formula called a structural formula may be needed. Structural formulas may in turn be represented with a one-dimensional chemical name, but such chemical nomenclature requires many words and terms which are not part of chemical formulas.
For molecules with a complicated 3-dimensional structure, especially involving atoms bonded to four different substituents, a simple molecular formula or even semi-structural chemical formula may not be enough to completely specify the molecule. In this case, a graphical type of formula called a structural formula may be needed. Structural formulas may in turn be represented with a one-dimensional chemical name, but such chemical nomenclature requires many words and terms which are not part of chemical formulas.
Molecular geometry
 Molecules have fixed equilibrium geometries—bond lengths and angles— about which they continuously oscillate through vibrational and rotational motions. A pure substance is composed of molecules with the same average geometrical structure. The chemical formula and the structure of a molecule are the two important factors that determine its properties, particularly its reactivity.
Molecules have fixed equilibrium geometries—bond lengths and angles— about which they continuously oscillate through vibrational and rotational motions. A pure substance is composed of molecules with the same average geometrical structure. The chemical formula and the structure of a molecule are the two important factors that determine its properties, particularly its reactivity. Isomers
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
...
share a chemical formula but normally have very different properties because of their different structures. Stereoisomer
In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in ...
s, a particular type of isomer, may have very similar physico-chemical properties and at the same time different biochemical activities.
Molecular spectroscopy
 Molecular spectroscopy deals with the response (
Molecular spectroscopy deals with the response (spectrum
A spectrum (plural ''spectra'' or ''spectrums'') is a condition that is not limited to a specific set of values but can vary, without gaps, across a continuum. The word was first used scientifically in optics to describe the rainbow of colors ...
) of molecules interacting with probing signals of known energy
In physics, energy (from Ancient Greek: ἐνέργεια, ''enérgeia'', “activity”) is the quantitative property that is transferred to a body or to a physical system, recognizable in the performance of work and in the form of hea ...
(or frequency
Frequency is the number of occurrences of a repeating event per unit of time. It is also occasionally referred to as ''temporal frequency'' for clarity, and is distinct from ''angular frequency''. Frequency is measured in hertz (Hz) which is eq ...
, according to Planck's formula). Molecules have quantized energy levels that can be analyzed by detecting the molecule's energy exchange through absorbance
Absorbance is defined as "the logarithm of the ratio of incident to transmitted radiant power through a sample (excluding the effects on cell walls)". Alternatively, for samples which scatter light, absorbance may be defined as "the negative lo ...
or emission.
Spectroscopy does not generally refer to diffraction studies where particles such as neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons beh ...
s, electrons, or high energy X-ray
An X-ray, or, much less commonly, X-radiation, is a penetrating form of high-energy electromagnetic radiation. Most X-rays have a wavelength ranging from 10 picometers to 10 nanometers, corresponding to frequencies in the range 30&nb ...
s interact with a regular arrangement of molecules (as in a crystal).
Microwave spectroscopy
Microwave spectroscopy is the spectroscopy method that employs microwaves, i.e. electromagnetic radiation at GHz frequencies, for the study of matter.
History
The ammonia molecule NH3 is shaped like a pyramid 0.38 Å in height, with an equilatera ...
commonly measures changes in the rotation of molecules, and can be used to identify molecules in outer space. Infrared spectroscopy
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or function ...
measures the vibration of molecules, including stretching, bending or twisting motions. It is commonly used to identify the kinds of bonds or functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the re ...
s in molecules. Changes in the arrangements of electrons yield absorption or emission lines in ultraviolet, visible or near infrared
Infrared (IR), sometimes called infrared light, is electromagnetic radiation (EMR) with wavelengths longer than those of visible light. It is therefore invisible to the human eye. IR is generally understood to encompass wavelengths from arou ...
light, and result in colour. Nuclear resonance spectroscopy measures the environment of particular nuclei in the molecule, and can be used to characterise the numbers of atoms in different positions in a molecule.
Theoretical aspects
The study of molecules by molecular physics andtheoretical chemistry
Theoretical chemistry is the branch of chemistry which develops theoretical generalizations that are part of the theoretical arsenal of modern chemistry: for example, the concepts of chemical bonding, chemical reaction, valence, the surface o ...
is largely based on quantum mechanic
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistry, qua ...
s and is essential for the understanding of the chemical bond. The simplest of molecules is the hydrogen molecule-ion
The dihydrogen cation or hydrogen molecular ion is a cation (positive ion) with formula . It consists of two hydrogen nuclei ( protons) sharing a single electron. It is the simplest molecular ion.
The ion can be formed from the ionization of a ...
, H2+, and the simplest of all the chemical bonds is the one-electron bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
. H2+ is composed of two positively charged protons and one negatively charged electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no ...
, which means that the Schrödinger equation
The Schrödinger equation is a linear partial differential equation that governs the wave function of a quantum-mechanical system. It is a key result in quantum mechanics, and its discovery was a significant landmark in the development of th ...
for the system can be solved more easily due to the lack of electron–electron repulsion. With the development of fast digital computers, approximate solutions for more complicated molecules became possible and are one of the main aspects of computational chemistry.
When trying to define rigorously whether an arrangement of atoms is ''sufficiently stable'' to be considered a molecule, IUPAC suggests that it "must correspond to a depression on the potential energy surface
A potential energy surface (PES) describes the energy of a system, especially a collection of atoms, in terms of certain parameters, normally the positions of the atoms. The surface might define the energy as a function of one or more coordinat ...
that is deep enough to confine at least one vibrational state". This definition does not depend on the nature of the interaction between the atoms, but only on the strength of the interaction. In fact, it includes weakly bound species that would not traditionally be considered molecules, such as the helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. ...
dimer
Dimer may refer to:
* Dimer (chemistry), a chemical structure formed from two similar sub-units
** Protein dimer, a protein quaternary structure
** d-dimer
* Dimer model, an item in statistical mechanics, based on ''domino tiling''
* Julius Dimer ...
, He2, which has one vibrational bound state and is so loosely bound that it is only likely to be observed at very low temperatures.
Whether or not an arrangement of atoms is ''sufficiently stable'' to be considered a molecule is inherently an operational definition. Philosophically, therefore, a molecule is not a fundamental entity (in contrast, for instance, to an elementary particle
In particle physics, an elementary particle or fundamental particle is a subatomic particle that is not composed of other particles. Particles currently thought to be elementary include electrons, the fundamental fermions ( quarks, leptons, a ...
); rather, the concept of a molecule is the chemist's way of making a useful statement about the strengths of atomic-scale interactions in the world that we observe.
See also
*Atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, ...
* Chemical polarity
* Chemical structure
A chemical structure determination includes a chemist's specifying the molecular geometry and, when feasible and necessary, the electronic structure of the target molecule or other solid. Molecular geometry refers to the spatial arrangement of ...
* Covalent bond
* Diatomic molecule
Diatomic molecules () are molecules composed of only two atoms, of the same or different chemical elements. If a diatomic molecule consists of two atoms of the same element, such as hydrogen () or oxygen (), then it is said to be homonuclear. O ...
* List of compounds
Compounds are organized into the following lists:
* , compounds without a C–H bond
*
See also
*
*
*
*
*
*
*
*
*
* can form compounds
External links
Relevant links for chemical compounds are:
* The CASbr>Substance DatabasesCo ...
* List of interstellar and circumstellar molecules
This is a list of molecules that have been detected in the interstellar medium and circumstellar envelopes, grouped by the number of component atoms. The chemical formula is listed for each detected compound, along with any ionized form that has ...
* Molecular biology
Molecular biology is the branch of biology that seeks to understand the molecular basis of biological activity in and between cells, including biomolecular synthesis, modification, mechanisms, and interactions. The study of chemical and physi ...
* Molecular design software Molecular design software is notable software for molecular modeling, that provides special support for developing molecular models ''de novo''.
In contrast to the usual molecular modeling programs, such as for molecular dynamics and quantum chemi ...
* Molecular engineering
Molecular engineering is an emerging field of study concerned with the design and testing of molecular properties, behavior and interactions in order to assemble better materials, systems, and processes for specific functions. This approach, in whi ...
* Molecular geometry
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that dete ...
* Molecular Hamiltonian
In atomic, molecular, and optical physics and quantum chemistry, the molecular Hamiltonian is the Hamiltonian operator representing the energy of the electrons and nuclei in a molecule. This operator and the associated Schrödinger equation p ...
* Molecular ion
Mass spectral interpretation is the method employed to identify the chemical formula, characteristic fragment patterns and possible fragment ions from the mass spectra. Mass spectra is a plot of relative abundance against mass-to-charge ratio. It i ...
* Molecular modelling
Molecular modelling encompasses all methods, theoretical and computational, used to model or mimic the behaviour of molecules. The methods are used in the fields of computational chemistry, drug design, computational biology and materials sci ...
* Molecular promiscuity
Molecular promiscuity indicates the ability of a molecule to bind to interact with one or more other classes and subtypes of molecules, in synergistic or antagonistic ways. These interactions may involve multiple paracrine, endocrine and autocrine ...
* Molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of findin ...
* Non-covalent bonding
* Periodic systems of small molecules
Periodic systems of molecules are charts of molecules similar to the periodic table of the elements. Construction of such charts was initiated in the early 20th century and is still ongoing.
It is commonly believed that the periodic law, represen ...
* Small molecule
Within the fields of molecular biology and pharmacology, a small molecule or micromolecule is a low molecular weight (≤ 1000 daltons) organic compound that may regulate a biological process, with a size on the order of 1 nm. Many drugs ...
* Comparison of software for molecular mechanics modeling
This is a list of computer programs that are predominantly used for molecular mechanics calculations.
See also
* Car–Parrinello molecular dynamics
* Comparison of force-field implementations
*Comparison of nucleic acid simulation software ...
* Van der Waals molecule
A Van der Waals molecule is a weakly bound complex of atoms or molecules held together by intermolecular attractions such as Van der Waals forces or by hydrogen bonds.
The name originated in the beginning of the 1970s when stable molecular clust ...
* World Wide Molecular Matrix
The World Wide Molecular Matrix (WWMM) was a proposed electronic repository for unpublished chemical data. First introduced in 2002 by Peter Murray-Rust and his colleagues in the chemistry department at the University of Cambridge in the United ...
References
External links
Molecule of the MonthSchool of Chemistry, University of Bristol
{{Authority control Chemistry Matter