Moenomycin A on:
[Wikipedia]
[Google]
[Amazon]
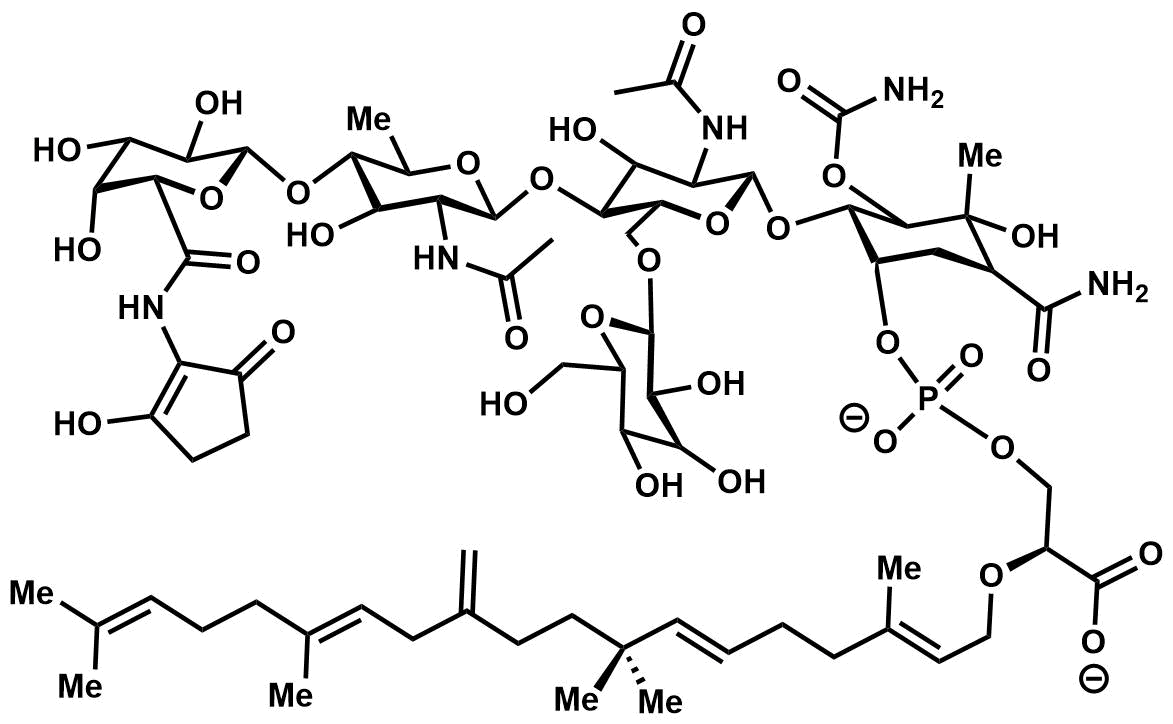 First described in 1965, the moenomycins are a family of phospho
First described in 1965, the moenomycins are a family of phospho
 The moenomycins can be reduced to three key structural features
# A central
The moenomycins can be reduced to three key structural features
# A central
 Extensive exploration into the
Extensive exploration into the
 First described in 1965, the moenomycins are a family of phospho
First described in 1965, the moenomycins are a family of phosphoglycolipid
Glycolipids are lipids with a carbohydrate attached by a glycosidic (covalent) bond. Their role is to maintain the stability of the cell membrane and to facilitate cellular recognition, which is crucial to the immune response and in the connec ...
antibiotics
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention o ...
, metabolites of the bacterial genus ''Streptomyces
''Streptomyces'' is the largest genus of Actinomycetota and the type genus of the family Streptomycetaceae. Over 500 species of ''Streptomyces'' bacteria have been described. As with the other Actinomycetota, streptomycetes are gram-positive, ...
''. Moenomycin A is the founding member of the antibiotic family with the majority discovered by the end of the late 1970s.
Structure
 The moenomycins can be reduced to three key structural features
# A central
The moenomycins can be reduced to three key structural features
# A central 3-phosphoglyceric acid
3-Phosphoglyceric acid (3PG, 3-PGA, or PGA) is the conjugate acid of 3-phosphoglycerate or glycerate 3-phosphate (GP or G3P). This glycerate is a biochemically significant metabolic intermediate in both glycolysis and the Calvin-Benson cycle. The ...
backbone.
# A 25-carbon isoprenoid
The terpenoids, also known as isoprenoids, are a class of naturally occurring organic chemicals derived from the 5-carbon compound isoprene and its derivatives called terpenes, diterpenes, etc. While sometimes used interchangeably with "terpenes", ...
chain connected by an ether linkage to the C2-position of 3-phosphoglyceric acid.
# A substituted tetra saccharide tethered via a phosphodiester
In chemistry, a phosphodiester bond occurs when exactly two of the hydroxyl groups () in phosphoric acid react with hydroxyl groups on other molecules to form two ester bonds. The "bond" involves this linkage . Discussion of phosphodiesters is d ...
linkage to 3-phosphoglyceric acid.
It is the combination of different isoprenoid chains and variously substituted tetrasaccharides that give rise to the diversity of the moenomycin family.
Based on degradation experiments, the defining mark of a moenomycin is the presence of the 25-carbon alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
moenocinol or diumycinol upon hydrolysis of the lipid tail; these alcohols originate from the L1 or L2 lipid respectively in the figure. These two structures are the only observed lipid tails within the moenomycin family, with AC326-α being the only known for producing diumycinol.
With regards to the tetrasaccharide portion, stereochemistry and functionality can differ at R1 and R2 depending on if this saccharide unit is D-gluco versus D-galacto; there is an axial methyl group in the former case with the exception of moenomycin A12 and C1 where there is instead an axial hydroxyl. The oligosaccharide motif can be deoxygenated, hydroxylated, or glycosylated at the R3 position – notable examples of the pentasaccharide motif include moenomycin A and AC326-α. It is believed the additional glycan
The terms glycans and polysaccharides are defined by IUPAC as synonyms meaning "compounds consisting of a large number of monosaccharides linked glycosidically". However, in practice the term glycan may also be used to refer to the carbohydrate p ...
can enhance specificity and binding to the target protein, affording increased activity. With the exception of pholipomycin and AC326-α, the R4 saccharide unit is usually the deoxysaccharide. Lastly, in the majority of moenomycins the R5 position is linked to a 2-aminocyclopentane-1,3-dione – a convenient chromophore
A chromophore is the part of a molecule responsible for its color.
The color that is seen by our eyes is the one not absorbed by the reflecting object within a certain wavelength spectrum of visible light. The chromophore is a region in the molec ...
utilized for structural analysis. For the nosokomycin subfamily, this position forms a carboxamide or carboxylic acid.Ostash, B.; Walker, S. Nat. Prod. Rep., 2010, 27, 1594-1617
Chemical synthesis
Due to the structural complexity of the moenomycins, total synthesis has proved difficult, with only one total synthesis reported so far. Some of the largest challenges include fashioning the glycosidic linkages withstereochemical
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereois ...
control and site-specifically decorating the oligosaccharide with pendant functionality. Understanding that the majority of variation within the moenomycin family derives from differences within the oligosaccharide unit, Kahne and lab has designed an efficient and flexible total synthesis of moenomycin A that gives access to analogues as well as other members of the moenomycin family.
Biosynthesis
 Extensive exploration into the
Extensive exploration into the biosynthesis
Biosynthesis is a multi-step, enzyme-catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined to form macromolecules. ...
of the moenomycin family has been conducted to better inform the genetic engineering
Genetic engineering, also called genetic modification or genetic manipulation, is the modification and manipulation of an organism's genes using technology. It is a set of technologies used to change the genetic makeup of cells, including t ...
and biosynthesis of novel moenomycin analogues. Early work on the biosynthesis of the moenomycins focused on the 25-carbon lipid tail derived from moenocinol; the tail was of particular interest given that it appears to break the isoprene rule
Terpenes () are a class of natural products consisting of compounds with the formula (C5H8)n for n > 1. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. Terpenes ar ...
at C8, containing a quaternary carbon. Feeding studies revealed the moenocinol lipid tail originates from a 15-carbon farnesyl
Farnesol is a natural 15-carbon organic compound which is an acyclic sesquiterpene alcohol. Under standard conditions, it is a colorless liquid. It is hydrophobic, and thus insoluble in water, but miscible with oils.
Farnesol is produced from 5- ...
precursor and a 10-carbon geranyl pyrophosphate.
More recently, the biosynthetic gene cluster for moenomycin A was first described in 2007 in ''Streptomyces ghanaensis
''Streptomyces viridosporus'' is a bacterium species from the genus of ''Streptomyces''.Deutsche Sammlung von Mikroorganismen und Zellkulturenbr>/ref> ''Streptomyces viridosporus'' produces sistomycine and lignin peroxidase. ''Streptomyces viri ...
''. In 2009, the seventeen step biosynthetic pathway was completely characterized, revealing the order of assembly for the molecular scaffold.
Medicinal use
The moenomycins target bacterial peptidoglycanglycosyltransferases
Glycosyltransferases (GTFs, Gtfs) are enzymes ( EC 2.4) that establish natural glycosidic linkages. They catalyze the transfer of saccharide moieties from an activated nucleotide sugar (also known as the "glycosyl donor") to a nucleophilic glycos ...
, inhibiting cell wall formation, leading to cell death. In general, the antibiotics are particularly potent against gram-positive bacteria
In bacteriology, gram-positive bacteria are bacteria that give a positive result in the Gram stain test, which is traditionally used to quickly classify bacteria into two broad categories according to their type of cell wall.
Gram-positive bact ...
with a minimum inhibitory concentration (MIC) between 1–100 (ng/ml). At higher concentrations the moenomycins are also effective against gram-negative bacteria with an MIC between 0.3–150 (μg/ml). ''In vivo'' studies using mice models suggest the antibiotics are powerful prophylactic and therapeutic agents, with subcutaneous injection being the most effective mode of delivery.
Moenomycins A and C are commercially used in the formulation of Bambermycins (Flavomycin), a veterinary antibiotic used solely in poultry, swine, and cattle feed.
Due to poor pharmacokinetic properties from the 25-carbon lipid chain, the moenomycins are not used in humans. The pharmacophore is well understood however, allowing the moenomycins to serve as the blueprint for future antibacterials.
Mode of action
General
The moenomycin family functions as an antibiotic by reversibly binding bacterial transglycosylases, essential enzymes that catalyze the extension of the glycan chain of the cell wall to form a stable peptidoglycan layer. The moenomycins mimic and thus compete with the natural substrate of the enzyme, inhibiting growth of the cell wall. Compromise of the wall results in leakage of cell contents, and ultimately cell death. The moenomycins are the only known active site inhibitors of these enzymes, which in lies their promise as human antibiotics given pathogenic bacteria have not yet widely evolvedresistance
Resistance may refer to:
Arts, entertainment, and media Comics
* Either of two similarly named but otherwise unrelated comic book series, both published by Wildstorm:
** ''Resistance'' (comics), based on the video game of the same title
** ''T ...
.
Structure-activity relationships
The 25-carbon lipid tail confers to the moenomycins a detergent-like property that allows them to become incorporated into the cytoplasmic membrane of the target bacterial cell. This anchoring presents the oligosaccharide portion of the molecule to the transglycosylase where it can tightly and selectively bind the enzyme, inhibiting cell wall growth. This property however undermines their use in clinical settings. The amphiphilic nature of the moenomycins induce hemolytic activity, provide a long half-life in the blood stream, and creates a tendency to aggregate in aqueous solution. Comparison of moenomycins with an abridged isoprene chain of 10-carbons, show that the oligosaccharide can still tightly bind the enzyme active site, but ''in vivo'' the MIC significantly increases since the drug is unable to anchor itself to the cytoplasmic membrane and present its sugar moiety. Further studies are needed to determine the optimal length for favorable pharmacokinetic properties. In contrast to the lipid portion, the oligosaccharide portion of the moenomycins is relatively well understood. When absent, the chromophore portion can decrease activity by 10-fold, suggesting it is not necessary for recognition but provides additional contacts with the target enzyme.T. Ru¨hl et al. / Bioorg. Med. Chem. 11 (2003) 2965–2981References
{{reflist Antibiotics