Maxwell–Boltzmann distribution on:
[Wikipedia]
[Google]
[Amazon]
In
/ref>Maxwell, J.C. (1860 B): ''Illustrations of the dynamical theory of gases. Part II. On the process of diffusion of two or more kinds of moving particles among one another. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science'', 4th Ser., vol.20, pp.21–37
/ref> Boltzmann later, in the 1870s, carried out significant investigations into the physical origins of this distribution. The distribution can be derived on the ground that it maximizes the entropy of the system. A list of derivations are: # Maximum entropy probability distribution#Distributions with measured constants, Maximum entropy probability distribution in the phase space, with the constraint of conservation of average energy #
 The
The
"The Maxwell Speed Distribution"
from The Wolfram Demonstrations Project at
physics
Physics is the scientific study of matter, its Elementary particle, fundamental constituents, its motion and behavior through space and time, and the related entities of energy and force. "Physical science is that department of knowledge whi ...
(in particular in statistical mechanics
In physics, statistical mechanics is a mathematical framework that applies statistical methods and probability theory to large assemblies of microscopic entities. Sometimes called statistical physics or statistical thermodynamics, its applicati ...
), the Maxwell–Boltzmann distribution, or Maxwell(ian) distribution, is a particular probability distribution
In probability theory and statistics, a probability distribution is a Function (mathematics), function that gives the probabilities of occurrence of possible events for an Experiment (probability theory), experiment. It is a mathematical descri ...
named after James Clerk Maxwell
James Clerk Maxwell (13 June 1831 – 5 November 1879) was a Scottish physicist and mathematician who was responsible for the classical theory of electromagnetic radiation, which was the first theory to describe electricity, magnetism an ...
and Ludwig Boltzmann
Ludwig Eduard Boltzmann ( ; ; 20 February 1844 – 5 September 1906) was an Austrian mathematician and Theoretical physics, theoretical physicist. His greatest achievements were the development of statistical mechanics and the statistical ex ...
.
It was first defined and used for describing particle speed
In kinematics, the speed (commonly referred to as ''v'') of an object is the magnitude of the change of its position over time or the magnitude of the change of its position per unit of time; it is thus a non-negative scalar quantity. Intro ...
s in idealized gases, where the particles move freely inside a stationary container without interacting with one another, except for very brief collision
In physics, a collision is any event in which two or more bodies exert forces on each other in a relatively short time. Although the most common use of the word ''collision'' refers to incidents in which two or more objects collide with great for ...
s in which they exchange energy and momentum with each other or with their thermal environment. The term "particle" in this context refers to gaseous particles only (atoms
Atoms are the basic particles of the chemical elements. An atom consists of a nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished from each other ...
or molecules
A molecule is a group of two or more atoms that are held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemistry ...
), and the system of particles is assumed to have reached thermodynamic equilibrium
Thermodynamic equilibrium is a notion of thermodynamics with axiomatic status referring to an internal state of a single thermodynamic system, or a relation between several thermodynamic systems connected by more or less permeable or impermeable ...
. The energies of such particles follow what is known as Maxwell–Boltzmann statistics
In statistical mechanics, Maxwell–Boltzmann statistics describes the distribution of classical material particles over various energy states in thermal equilibrium. It is applicable when the temperature is high enough or the particle density ...
, and the statistical distribution of speeds is derived by equating particle energies with kinetic energy
In physics, the kinetic energy of an object is the form of energy that it possesses due to its motion.
In classical mechanics, the kinetic energy of a non-rotating object of mass ''m'' traveling at a speed ''v'' is \fracmv^2.Resnick, Rober ...
.
Mathematically, the Maxwell–Boltzmann distribution is the chi distribution
In probability theory and statistics, the chi distribution is a continuous probability distribution over the non-negative real line. It is the distribution of the positive square root of a sum of squared independent Gaussian random variables. E ...
with three degrees of freedom
In many scientific fields, the degrees of freedom of a system is the number of parameters of the system that may vary independently. For example, a point in the plane has two degrees of freedom for translation: its two coordinates; a non-infinite ...
(the components of the velocity
Velocity is a measurement of speed in a certain direction of motion. It is a fundamental concept in kinematics, the branch of classical mechanics that describes the motion of physical objects. Velocity is a vector (geometry), vector Physical q ...
vector in Euclidean space
Euclidean space is the fundamental space of geometry, intended to represent physical space. Originally, in Euclid's ''Elements'', it was the three-dimensional space of Euclidean geometry, but in modern mathematics there are ''Euclidean spaces ...
), with a scale parameter
In probability theory and statistics, a scale parameter is a special kind of numerical parameter of a parametric family of probability distributions. The larger the scale parameter, the more spread out the distribution.
Definition
If a family ...
measuring speeds in units proportional to the square root of (the ratio of temperature and particle mass).
The Maxwell–Boltzmann distribution is a result of the kinetic theory of gases
The kinetic theory of gases is a simple classical model of the thermodynamic behavior of gases. Its introduction allowed many principal concepts of thermodynamics to be established. It treats a gas as composed of numerous particles, too small ...
, which provides a simplified explanation of many fundamental gaseous properties, including pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and eve ...
and diffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical p ...
. The Maxwell–Boltzmann distribution applies fundamentally to particle velocities in three dimensions, but turns out to depend only on the speed (the magnitude of the velocity) of the particles. A particle speed probability distribution indicates which speeds are more likely: a randomly chosen particle will have a speed selected randomly from the distribution, and is more likely to be within one range of speeds than another. The kinetic theory of gases applies to the classical ideal gas
An ideal gas is a theoretical gas composed of many randomly moving point particles that are not subject to interparticle interactions. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is ...
, which is an idealization of real gases. In real gases, there are various effects (e.g., van der Waals interactions, vortical flow, relativistic speed limits, and quantum exchange interaction
In chemistry and physics, the exchange interaction is a quantum mechanical constraint on the states of indistinguishable particles. While sometimes called an exchange force, or, in the case of fermions, Pauli repulsion, its consequences cannot alw ...
s) that can make their speed distribution different from the Maxwell–Boltzmann form. However, rarefied gases at ordinary temperatures behave very nearly like an ideal gas and the Maxwell speed distribution is an excellent approximation for such gases. This is also true for ideal plasmas, which are ionized gases of sufficiently low density.
The distribution was first derived by Maxwell in 1860 on heuristic
A heuristic or heuristic technique (''problem solving'', '' mental shortcut'', ''rule of thumb'') is any approach to problem solving that employs a pragmatic method that is not fully optimized, perfected, or rationalized, but is nevertheless ...
grounds.Maxwell, J.C. (1860 A): ''Illustrations of the dynamical theory of gases. Part I. On the motions and collisions of perfectly elastic spheres. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science'', 4th Series, vol.19, pp.19–32/ref>Maxwell, J.C. (1860 B): ''Illustrations of the dynamical theory of gases. Part II. On the process of diffusion of two or more kinds of moving particles among one another. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science'', 4th Ser., vol.20, pp.21–37
/ref> Boltzmann later, in the 1870s, carried out significant investigations into the physical origins of this distribution. The distribution can be derived on the ground that it maximizes the entropy of the system. A list of derivations are: # Maximum entropy probability distribution#Distributions with measured constants, Maximum entropy probability distribution in the phase space, with the constraint of conservation of average energy #
Canonical ensemble
In statistical mechanics, a canonical ensemble is the statistical ensemble that represents the possible states of a mechanical system in thermal equilibrium with a heat bath at a fixed temperature. The system can exchange energy with the hea ...
.
Distribution function
For a system containing a large number of identical non-interacting, non-relativistic classical particles in thermodynamic equilibrium, the fraction of the particles within an infinitesimal element of the three-dimensional velocity space , centered on a velocity vector of magnitude , is given by where: * is the particle mass; * is theBoltzmann constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative thermal energy of particles in a ideal gas, gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin (K) and the ...
;
* is thermodynamic temperature
Thermodynamic temperature, also known as absolute temperature, is a physical quantity which measures temperature starting from absolute zero, the point at which particles have minimal thermal motion.
Thermodynamic temperature is typically expres ...
;
* is a probability distribution function, properly normalized so that over all velocities is unity.
One can write the element of velocity space as , for velocities in a standard Cartesian coordinate system, or as in a standard spherical coordinate system, where is an element of solid angle and .
The Maxwellian distribution function for particles moving in only one direction, if this direction is , is
which can be obtained by integrating the three-dimensional form given above over and .
Recognizing the symmetry of , one can integrate over solid angle and write a probability distribution of speeds as the function
This probability density function
In probability theory, a probability density function (PDF), density function, or density of an absolutely continuous random variable, is a Function (mathematics), function whose value at any given sample (or point) in the sample space (the s ...
gives the probability, per unit speed, of finding the particle with a speed near . This equation is simply the Maxwell–Boltzmann distribution (given in the infobox) with distribution parameter
The Maxwell–Boltzmann distribution is equivalent to the chi distribution
In probability theory and statistics, the chi distribution is a continuous probability distribution over the non-negative real line. It is the distribution of the positive square root of a sum of squared independent Gaussian random variables. E ...
with three degrees of freedom and scale parameter
In probability theory and statistics, a scale parameter is a special kind of numerical parameter of a parametric family of probability distributions. The larger the scale parameter, the more spread out the distribution.
Definition
If a family ...
The simplest ordinary differential equation
In mathematics, an ordinary differential equation (ODE) is a differential equation (DE) dependent on only a single independent variable (mathematics), variable. As with any other DE, its unknown(s) consists of one (or more) Function (mathematic ...
satisfied by the distribution is:
or in unitless presentation:
With the Darwin–Fowler method of mean values, the Maxwell–Boltzmann distribution is obtained as an exact result.

Relaxation to the 2D Maxwell–Boltzmann distribution
For particles confined to move in a plane, the speed distribution is given by This distribution is used for describing systems in equilibrium. However, most systems do not start out in their equilibrium state. The evolution of a system towards its equilibrium state is governed by theBoltzmann equation
The Boltzmann equation or Boltzmann transport equation (BTE) describes the statistical behaviour of a thermodynamic system not in a state of equilibrium; it was devised by Ludwig Boltzmann in 1872.Encyclopaedia of Physics (2nd Edition), R. G ...
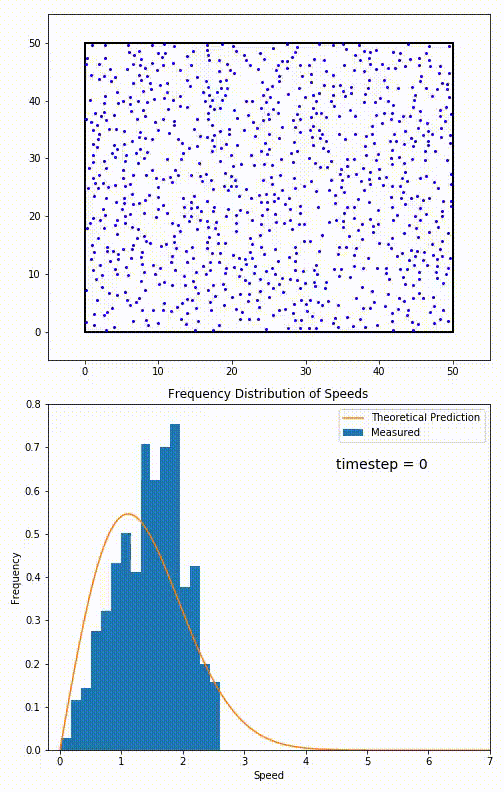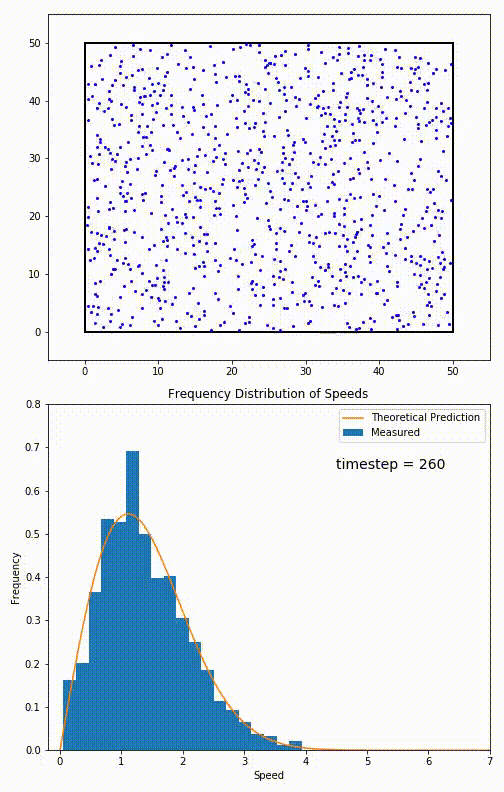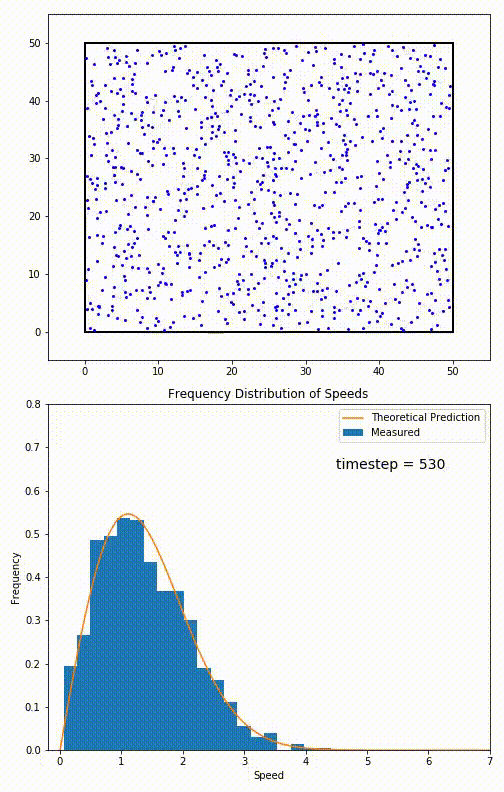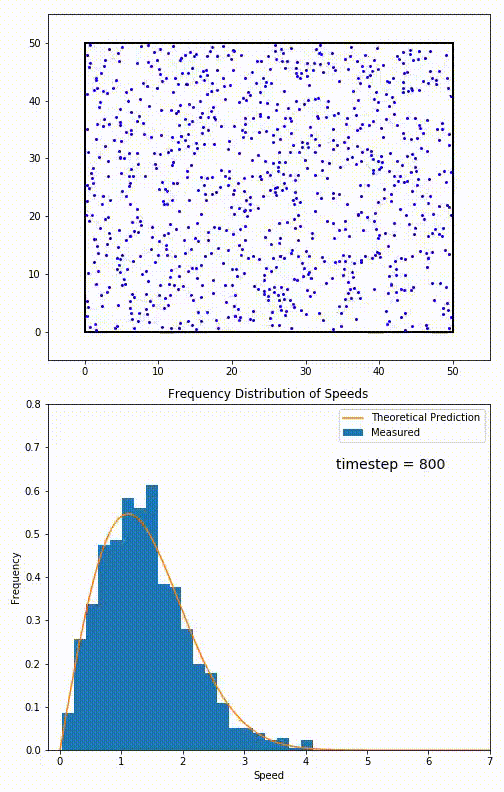
. The equation predicts that for short range interactions, the equilibrium velocity distribution will follow a Maxwell–Boltzmann distribution. To the right is a molecular dynamics
Molecular dynamics (MD) is a computer simulation method for analyzing the Motion (physics), physical movements of atoms and molecules. The atoms and molecules are allowed to interact for a fixed period of time, giving a view of the dynamics ( ...
(MD) simulation in which 900 hard sphere particles are constrained to move in a rectangle. They interact via perfectly elastic collisions. The system is initialized out of equilibrium, but the velocity distribution (in blue) quickly converges to the 2D Maxwell–Boltzmann distribution (in orange).
Typical speeds
 The
The mean
A mean is a quantity representing the "center" of a collection of numbers and is intermediate to the extreme values of the set of numbers. There are several kinds of means (or "measures of central tendency") in mathematics, especially in statist ...
speed , most probable speed ( mode) , and root-mean-square speed can be obtained from properties of the Maxwell distribution.
This works well for nearly ideal, monatomic
In physics and chemistry, "monatomic" is a combination of the words "mono" and "atomic", and means "single atom". It is usually applied to gases: a monatomic gas is a gas in which atoms are not bound to each other. Examples at standard conditions ...
gases like helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is ...
, but also for molecular gases like diatomic oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
. This is because despite the larger heat capacity
Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change in its temperature. The SI unit of heat capacity is joule per kelvin (J/K).
Heat capacity is a ...
(larger internal energy at the same temperature) due to their larger number of degrees of freedom
In many scientific fields, the degrees of freedom of a system is the number of parameters of the system that may vary independently. For example, a point in the plane has two degrees of freedom for translation: its two coordinates; a non-infinite ...
, their translational kinetic energy
In physics, the kinetic energy of an object is the form of energy that it possesses due to its motion.
In classical mechanics, the kinetic energy of a non-rotating object of mass ''m'' traveling at a speed ''v'' is \fracmv^2.Resnick, Rober ...
(and thus their speed) is unchanged.
In summary, the typical speeds are related as follows:
The root mean square speed is directly related to the speed of sound
The speed of sound is the distance travelled per unit of time by a sound wave as it propagates through an elasticity (solid mechanics), elastic medium. More simply, the speed of sound is how fast vibrations travel. At , the speed of sound in a ...
in the gas, by
where is the adiabatic index, is the number of degrees of freedom
In many scientific fields, the degrees of freedom of a system is the number of parameters of the system that may vary independently. For example, a point in the plane has two degrees of freedom for translation: its two coordinates; a non-infinite ...
of the individual gas molecule. For the example above, diatomic nitrogen (approximating air
An atmosphere () is a layer of gases that envelop an astronomical object, held in place by the gravity of the object. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosph ...
) at , Nitrogen at room temperature is considered a "rigid" diatomic gas, with two rotational degrees of freedom additional to the three translational ones, and the vibrational degree of freedom not accessible. and
the true value for air can be approximated by using the average molar weight of air
An atmosphere () is a layer of gases that envelop an astronomical object, held in place by the gravity of the object. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosph ...
(), yielding at (corrections for variable humidity
Humidity is the concentration of water vapor present in the air. Water vapor, the gaseous state of water, is generally invisible to the human eye. Humidity indicates the likelihood for precipitation (meteorology), precipitation, dew, or fog t ...
are of the order of 0.1% to 0.6%).
The average relative velocity
where the three-dimensional velocity distribution is
The integral can easily be done by changing to coordinates and
Limitations
The Maxwell–Boltzmann distribution assumes that the velocities of individual particles are much less than the speed of light, i.e. that . For electrons, the temperature of electrons must be . For distribution of speeds of relativistic particles, see Maxwell–Jüttner distribution.Derivation and related distributions
Maxwell–Boltzmann statistics
The original derivation in 1860 byJames Clerk Maxwell
James Clerk Maxwell (13 June 1831 – 5 November 1879) was a Scottish physicist and mathematician who was responsible for the classical theory of electromagnetic radiation, which was the first theory to describe electricity, magnetism an ...
was an argument based on molecular collisions of the Kinetic theory of gases
The kinetic theory of gases is a simple classical model of the thermodynamic behavior of gases. Its introduction allowed many principal concepts of thermodynamics to be established. It treats a gas as composed of numerous particles, too small ...
as well as certain symmetries in the speed distribution function; Maxwell also gave an early argument that these molecular collisions entail a tendency towards equilibrium. After Maxwell, Ludwig Boltzmann
Ludwig Eduard Boltzmann ( ; ; 20 February 1844 – 5 September 1906) was an Austrian mathematician and Theoretical physics, theoretical physicist. His greatest achievements were the development of statistical mechanics and the statistical ex ...
in 1872 also derived the distribution on mechanical grounds and argued that gases should over time tend toward this distribution, due to collisions (see H-theorem). He later (1877) derived the distribution again under the framework of statistical thermodynamics. The derivations in this section are along the lines of Boltzmann's 1877 derivation, starting with result known as Maxwell–Boltzmann statistics
In statistical mechanics, Maxwell–Boltzmann statistics describes the distribution of classical material particles over various energy states in thermal equilibrium. It is applicable when the temperature is high enough or the particle density ...
(from statistical thermodynamics). Maxwell–Boltzmann statistics gives the average number of particles found in a given single-particle microstate
A microstate or ministate is a sovereign state having a very small population or land area, usually both. However, the meanings of "state" and "very small" are not well-defined in international law. Some recent attempts to define microstates ...
. Under certain assumptions, the logarithm of the fraction of particles in a given microstate is linear in the ratio of the energy of that state to the temperature of the system: there are constants and such that, for all ,
The assumptions of this equation are that the particles do not interact, and that they are classical; this means that each particle's state can be considered independently from the other particles' states. Additionally, the particles are assumed to be in thermal equilibrium.
This relation can be written as an equation by introducing a normalizing factor:
where:
* is the expected number of particles in the single-particle microstate ,
* is the total number of particles in the system,
* is the energy of microstate ,
* the sum over index takes into account all microstates,
* is the equilibrium temperature of the system,
* is the Boltzmann constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative thermal energy of particles in a ideal gas, gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin (K) and the ...
.
The denominator in is a normalizing factor so that the ratios add up to unity — in other words it is a kind of partition function (for the single-particle system, not the usual partition function of the entire system).
Because velocity and speed are related to energy, Equation () can be used to derive relationships between temperature and the speeds of gas particles. All that is needed is to discover the density of microstates in energy, which is determined by dividing up momentum space into equal sized regions.
Distribution for the momentum vector
The potential energy is taken to be zero, so that all energy is in the form of kinetic energy. The relationship between kinetic energy and momentum for massive non- relativistic particles is where is the square of the momentum vector . We may therefore rewrite Equation () as: where: * is the partition function, corresponding to the denominator in ; * is the molecular mass of the gas; * is the thermodynamic temperature; * is theBoltzmann constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative thermal energy of particles in a ideal gas, gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin (K) and the ...
.
This distribution of is proportional to the probability density function
In probability theory, a probability density function (PDF), density function, or density of an absolutely continuous random variable, is a Function (mathematics), function whose value at any given sample (or point) in the sample space (the s ...
for finding a molecule with these values of momentum components, so:
The normalizing constant
In probability theory, a normalizing constant or normalizing factor is used to reduce any probability function to a probability density function with total probability of one.
For example, a Gaussian function can be normalized into a probabilit ...
can be determined by recognizing that the probability of a molecule having ''some'' momentum must be 1.
Integrating the exponential in over all , , and yields a factor of
So that the normalized distribution function is:
The distribution is seen to be the product of three independent normally distributed
In probability theory and statistics, a normal distribution or Gaussian distribution is a type of continuous probability distribution for a real number, real-valued random variable. The general form of its probability density function is
f(x ...
variables , , and , with variance . Additionally, it can be seen that the magnitude of momentum will be distributed as a Maxwell–Boltzmann distribution, with . The Maxwell–Boltzmann distribution for the momentum (or equally for the velocities) can be obtained more fundamentally using the H-theorem at equilibrium within the Kinetic theory of gases
The kinetic theory of gases is a simple classical model of the thermodynamic behavior of gases. Its introduction allowed many principal concepts of thermodynamics to be established. It treats a gas as composed of numerous particles, too small ...
framework.
Distribution for the energy
The energy distribution is found imposing where is the infinitesimal phase-space volume of momenta corresponding to the energy interval . Making use of the spherical symmetry of the energy-momentum dispersion relation this can be expressed in terms of as Using then () in (), and expressing everything in terms of the energy , we get and finally Since the energy is proportional to the sum of the squares of the three normally distributed momentum components, this energy distribution can be written equivalently as a gamma distribution, using a shape parameter, and a scale parameter, Using theequipartition theorem
In classical physics, classical statistical mechanics, the equipartition theorem relates the temperature of a system to its average energy, energies. The equipartition theorem is also known as the law of equipartition, equipartition of energy, ...
, given that the energy is evenly distributed among all three degrees of freedom in equilibrium, we can also split into a set of chi-squared distribution
In probability theory and statistics, the \chi^2-distribution with k Degrees of freedom (statistics), degrees of freedom is the distribution of a sum of the squares of k Independence (probability theory), independent standard normal random vari ...
s, where the energy per degree of freedom, is distributed as a chi-squared distribution with one degree of freedom,
At equilibrium, this distribution will hold true for any number of degrees of freedom. For example, if the particles are rigid mass dipoles of fixed dipole moment, they will have three translational degrees of freedom and two additional rotational degrees of freedom. The energy in each degree of freedom will be described according to the above chi-squared distribution with one degree of freedom, and the total energy will be distributed according to a chi-squared distribution with five degrees of freedom. This has implications in the theory of the specific heat
In thermodynamics, the specific heat capacity (symbol ) of a substance is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit in temperature. It is also referred to as massic heat ...
of a gas.
Distribution for the velocity vector
Recognizing that the velocity probability density is proportional to the momentum probability density function by and using we get which is the Maxwell–Boltzmann velocity distribution. The probability of finding a particle with velocity in the infinitesimal element about velocity is Like the momentum, this distribution is seen to be the product of three independentnormally distributed
In probability theory and statistics, a normal distribution or Gaussian distribution is a type of continuous probability distribution for a real number, real-valued random variable. The general form of its probability density function is
f(x ...
variables , , and , but with variance .
It can also be seen that the Maxwell–Boltzmann velocity distribution for the vector velocity
is the product of the distributions for each of the three directions:
where the distribution for a single direction is
Each component of the velocity vector has a normal distribution
In probability theory and statistics, a normal distribution or Gaussian distribution is a type of continuous probability distribution for a real-valued random variable. The general form of its probability density function is
f(x) = \frac ...
with mean and standard deviation , so the vector has a 3-dimensional normal distribution, a particular kind of multivariate normal distribution
In probability theory and statistics, the multivariate normal distribution, multivariate Gaussian distribution, or joint normal distribution is a generalization of the one-dimensional ( univariate) normal distribution to higher dimensions. One d ...
, with mean and covariance , where is the identity matrix.
Distribution for the speed
The Maxwell–Boltzmann distribution for the speed follows immediately from the distribution of the velocity vector, above. Note that the speed is and thevolume element
In mathematics, a volume element provides a means for integrating a function with respect to volume in various coordinate systems such as spherical coordinates and cylindrical coordinates. Thus a volume element is an expression of the form
\ma ...
in spherical coordinates
In mathematics, a spherical coordinate system specifies a given point in three-dimensional space by using a distance and two angles as its three coordinates. These are
* the radial distance along the line connecting the point to a fixed point ...
where and are the spherical coordinate angles of the velocity vector. Integration of the probability density function of the velocity over the solid angles yields an additional factor of .
The speed distribution with substitution of the speed for the sum of the squares of the vector components:
In ''n''-dimensional space
In -dimensional space, Maxwell–Boltzmann distribution becomes: Speed distribution becomes: where is a normalizing constant. The following integral result is useful: where is theGamma function
In mathematics, the gamma function (represented by Γ, capital Greek alphabet, Greek letter gamma) is the most common extension of the factorial function to complex numbers. Derived by Daniel Bernoulli, the gamma function \Gamma(z) is defined ...
. This result can be used to calculate the moments of speed distribution function:
which is the mean
A mean is a quantity representing the "center" of a collection of numbers and is intermediate to the extreme values of the set of numbers. There are several kinds of means (or "measures of central tendency") in mathematics, especially in statist ...
speed itself
which gives root-mean-square speed
The derivative of speed distribution function:
This yields the most probable speed ( mode)
See also
* Quantum Boltzmann equation *Maxwell–Boltzmann statistics
In statistical mechanics, Maxwell–Boltzmann statistics describes the distribution of classical material particles over various energy states in thermal equilibrium. It is applicable when the temperature is high enough or the particle density ...
* Maxwell–Jüttner distribution
* Boltzmann distribution
In statistical mechanics and mathematics, a Boltzmann distribution (also called Gibbs distribution Translated by J.B. Sykes and M.J. Kearsley. See section 28) is a probability distribution or probability measure that gives the probability tha ...
* Rayleigh distribution
* Kinetic theory of gases
The kinetic theory of gases is a simple classical model of the thermodynamic behavior of gases. Its introduction allowed many principal concepts of thermodynamics to be established. It treats a gas as composed of numerous particles, too small ...
Notes
References
Further reading
* * * * * *External links
"The Maxwell Speed Distribution"
from The Wolfram Demonstrations Project at
Mathworld
''MathWorld'' is an online mathematics reference work, created and largely written by Eric W. Weisstein. It is sponsored by and licensed to Wolfram Research, Inc. and was partially funded by the National Science Foundation's National Science ...
{{DEFAULTSORT:Maxwell-Boltzmann Distribution
Continuous distributions
Gases
Ludwig Boltzmann
James Clerk Maxwell
Normal distribution
Particle distributions