Martin Sulfurane on:
[Wikipedia]
[Google]
[Amazon]
Martin's sulfurane is the 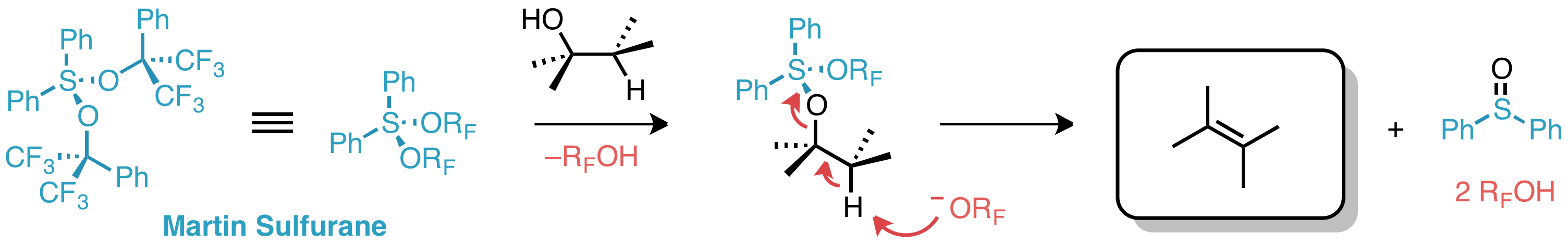
organosulfur compound
Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfu ...
with the formula Ph2S C(CF3)2Phsub>2 (Ph = C6H5). It is a white solid that easily undergoes sublimation
Sublimation or sublimate may refer to:
* ''Sublimation'' (album), by Canvas Solaris, 2004
* Sublimation (phase transition), directly from the solid to the gas phase
* Sublimation (psychology), a mature type of defense mechanism
* Sublimate of mer ...
. The compound is an example of a hypervalent sulfur compound called a sulfurane
Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur ...
. As such, the sulfur adopts a see-saw structure, with a lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
of electrons as the equatorial fifth coordinate of a trigonal bipyramid
In geometry, the triangular bipyramid (or dipyramid) is a type of hexahedron, being the first in the infinite set of face-transitive bipyramids. It is the dual of the triangular prism with 6 isosceles triangle faces.
As the name suggests, i ...
, like that of sulfur tetrafluoride
Sulfur tetrafluoride is the chemical compound with the formula S F4. It is a colorless corrosive gas that releases dangerous HF upon exposure to water or moisture. Despite these unwelcome characteristics, this compound is a useful reagent for t ...
(SF4). The compound is a reagent in organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
. One application is for the dehydration of a secondary alcohol to give an alkene:
:RCH(OH)CH2R' + Ph2S C(CF3)2Phsub>2 → RCH=CHR' + Ph2SO + 2 HOC(CF3)2Ph

References