Martin's Sulfurane on:
[Wikipedia]
[Google]
[Amazon]
Martin's sulfurane is the 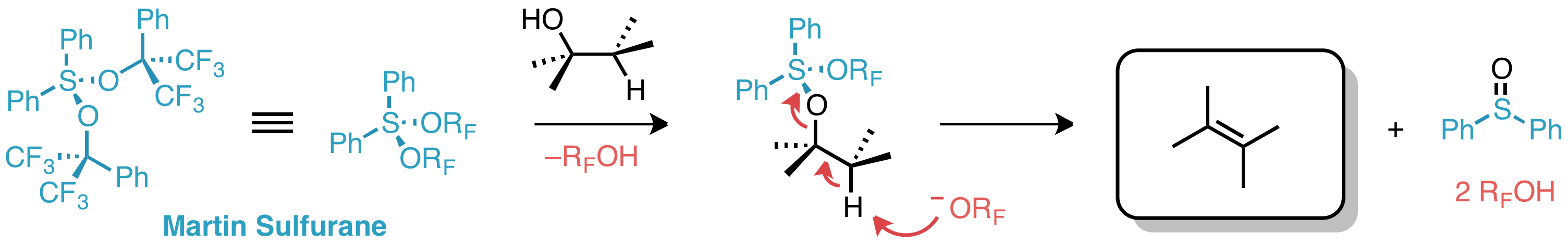
organosulfur compound
Organosulfur chemistry is the study of the properties and synthesis of organosulfur compounds, which are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur der ...
with the formula Ph2S C(CF3)2Phsub>2 (Ph = C6H5). It is a white solid that easily undergoes sublimation. The compound is an example of a hypervalent sulfur compound called a sulfurane. As such, the sulfur adopts a see-saw structure, with a lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
of electrons as the equatorial fifth coordinate of a trigonal bipyramid, like that of sulfur tetrafluoride (SF4). The compound is a reagent in organic synthesis
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the gen ...
. One application is for the dehydration
In physiology, dehydration is a lack of total body water that disrupts metabolic processes. It occurs when free water loss exceeds intake, often resulting from excessive sweating, health conditions, or inadequate consumption of water. Mild deh ...
of a secondary alcohol
In chemistry, an alcohol (), is a type of organic compound that carries at least one hydroxyl () functional group bound to a Saturated and unsaturated compounds, saturated carbon atom. Alcohols range from the simple, like methanol and ethanol ...
to give an alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
:
:RCH(OH)CH2R' + Ph2S C(CF3)2Phsub>2 → RCH=CHR' + Ph2SO + 2 HOC(CF3)2Ph

References