Manganese(II) Sulphate on:
[Wikipedia]
[Google]
[Amazon]
Manganese(II) sulfate usually refers to the
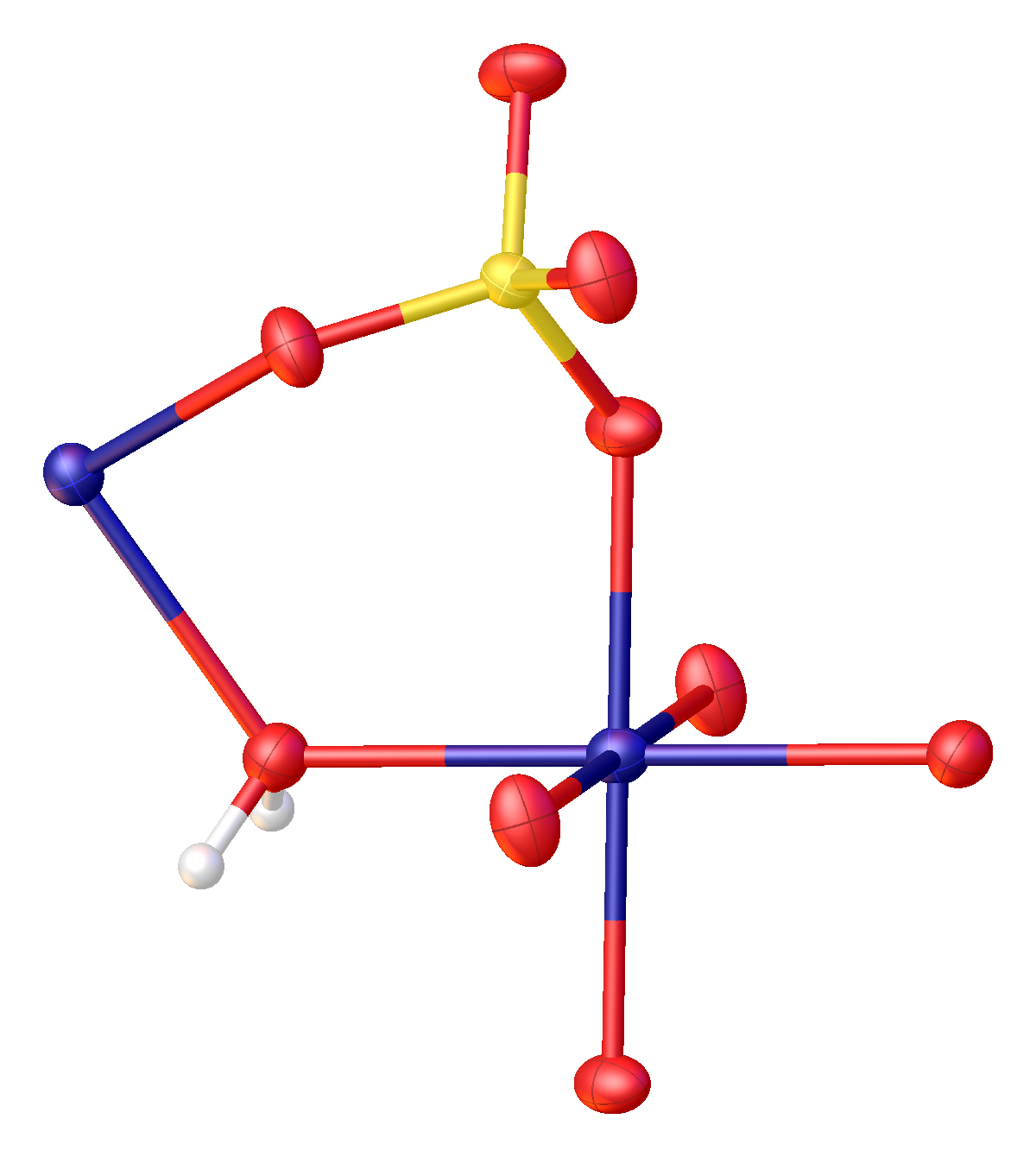 The structure of MnSO4·H2O has been determined by
The structure of MnSO4·H2O has been determined by
inorganic compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemist ...
with the formula
In science, a formula is a concise way of expressing information symbolically, as in a mathematical formula or a ''chemical formula''. The informal use of the term ''formula'' in science refers to the general construct of a relationship betwee ...
MnSO4·H2O. This pale pink deliquescent solid is a commercially significant manganese(II) salt. Approximately 260,000 tonne
The tonne ( or ; symbol: t) is a unit of mass equal to 1000 kilograms. It is a non-SI unit accepted for use with SI. It is also referred to as a metric ton to distinguish it from the non-metric units of the short ton ( United State ...
s of manganese(II) sulfate were produced worldwide in 2005. It is the precursor to manganese metal and many other chemical compounds. Manganese-deficient soil is remediated with this salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quantitie ...
.
Structure
 The structure of MnSO4·H2O has been determined by
The structure of MnSO4·H2O has been determined by X-ray crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
. Like many metal sulfates, manganese sulfate forms a variety of hydrates: monohydrate, tetrahydrate, pentahydrate, and heptahydrate. All of these salts dissolve in water to give faintly pink solutions of the aquo complex n(H2O)6sup>2+.
Applications and production
Typically, manganese ores are purified by their conversion to manganese(II) sulfate. Treatment of aqueous solutions of the sulfate withsodium carbonate
Sodium carbonate, , (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield moderately alkaline solutions ...
leads to precipitation of manganese carbonate
Manganese carbonate is a compound with the chemical formula Mn CO3. Manganese carbonate occurs naturally as the mineral rhodochrosite but it is typically produced industrially. It is a pale pink, water-insoluble solid. Approximately 20,000 metr ...
, which can be calcined to give the oxides MnO
Manganese(II) oxide is an inorganic compound with chemical formula MnO.Arno H. Reidies "Manganese Compounds" Ullmann's Encyclopedia of Chemical Technology 2007; Wiley-VCH, Weinheim. It forms green crystals. The compound is produced on a large ...
''x''. In the laboratory, manganese sulfate can be made by treating manganese dioxide
Manganese dioxide is the inorganic compound with the formula . This blackish or brown solid occurs naturally as the mineral pyrolusite, which is the main ore of manganese and a component of manganese nodules. The principal use for is for dry-cell ...
with sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic activ ...
:
:MnO2 + SO2 + H2O → MnSO4(H2O)
It can also be made by mixing potassium permanganate with sodium bisulfate
Sodium bisulfate, also known as sodium hydrogen sulfate, is the sodium salt of the bisulfate anion, with the molecular formula NaHSO4. Sodium bisulfate is an acid salt formed by partial neutralization of sulfuric acid by an equivalent of sodium b ...
and hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%†...
.
Manganese sulfate is a by-product of various industrially significant oxidations that use manganese dioxide, including the manufacture of hydroquinone
Hydroquinone, also known as benzene-1,4-diol or quinol, is an aromatic organic compound that is a type of phenol, a derivative of benzene, having the chemical formula C6H4(OH)2. It has two hydroxyl groups bonded to a benzene ring in a ''para'' ...
and anisaldehyde.
Electrolysis
Electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from n ...
of manganese sulfate reverses the above reaction yielding manganese dioxide
Manganese dioxide is the inorganic compound with the formula . This blackish or brown solid occurs naturally as the mineral pyrolusite, which is the main ore of manganese and a component of manganese nodules. The principal use for is for dry-cell ...
, which is called EMD for electrolytic manganese dioxide. Alternatively oxidation of manganese sulfate with potassium permanganate yields the so-called chemical manganese dioxide (CMD). These materials, especially EMD, are used in dry-cell batteries.
Natural occurrence
Manganese(II) sulfate minerals are very rare in nature and always occur as hydrates. The monohydrate is called szmikite; tetrahydrate = ilesite; hexahydrate (the most rare) = chvaleticeite; pentahydrate = jĹŤkokuite; heptahydrate = mallardite.References
{{Sulfates Manganese(II) compounds Sulfates Deliquescent substances