Magnesium-40 on:
[Wikipedia]
[Google]
[Amazon]
Magnesium isotopes data from ''The Berkeley Laboratory Isotopes Project's''
Magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ta ...
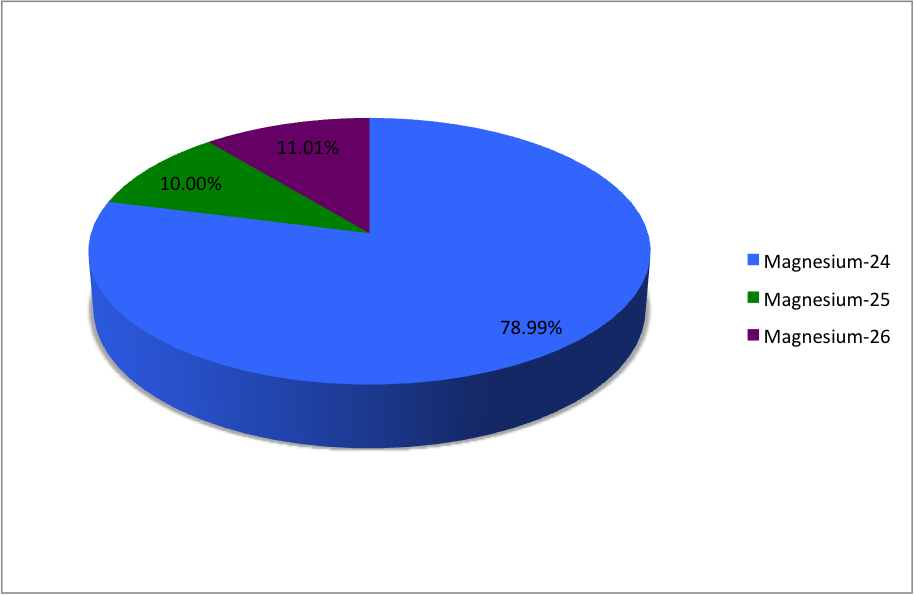
(12Mg) naturally occurs in three stable isotopes: , , and . There are 19 radioisotopes that have been discovered, ranging from to . The longest-lived radioisotope is with a half-life of . The lighter isotopes mostly decay to isotopes of sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable iso ...
while the heavier isotopes decay to isotopes of aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. I ...
. The shortest-lived is proton-unbound with a half-life of , though the half-life of similarly unbound has not been measured.

List of isotopes
, - , , style="text-align:right" , 12 , style="text-align:right" , 6 , , , 2p , , 0+ , , , - , , style="text-align:right" , 12 , style="text-align:right" , 7 , , , 2p , , 1/2−# , , , - , rowspan=2, , rowspan=2 style="text-align:right" , 12 , rowspan=2 style="text-align:right" , 8 , rowspan=2, , rowspan=2, , β+ () , , rowspan=2, 0+ , rowspan=2, , rowspan=2, , - , β+p () , , - , rowspan=4, , rowspan=4 style="text-align:right" , 12 , rowspan=4 style="text-align:right" , 9 , rowspan=4, , rowspan=4, , β+ () , , rowspan=4, 5/2+ , rowspan=4, , rowspan=4, , - , β+p () , , - , β+α () , , - , β+pα () , , - , , style="text-align:right" , 12 , style="text-align:right" , 10 , , , β+ , , 0+ , , , - , , style="text-align:right" , 12 , style="text-align:right" , 11 , , , β+ , , 3/2+ , , , - , , style="text-align:right" , 12 , style="text-align:right" , 12 , , colspan=3 align=center, Stable , 0+ , colspan=2 align=center, , - , , style="text-align:right" , 12 , style="text-align:right" , 13 , , colspan=3 align=center, Stable , 5/2+ , colspan=2 align=center, , - , Used inradiodating
Radiometric dating, radioactive dating or radioisotope dating is a technique which is used to date materials such as rocks or carbon, in which trace radioactive impurities were selectively incorporated when they were formed. The method compares t ...
events early in the Solar System's history
, style="text-align:right" , 12
, style="text-align:right" , 14
,
, colspan=3 align=center, Stable
, 0+
, colspan=2 align=center, , -
,
, style="text-align:right" , 12
, style="text-align:right" , 15
,
,
, β−
,
, 1/2+
,
,
, -
,
, style="text-align:right" , 12
, style="text-align:right" , 16
,
,
, β−
,
, 0+
,
,
, -
,
, style="text-align:right" , 12
, style="text-align:right" , 17
,
,
, β−
,
, 3/2+
,
,
, -
, rowspan=2,
, rowspan=2 style="text-align:right" , 12
, rowspan=2 style="text-align:right" , 18
, rowspan=2,
, rowspan=2,
, β− (> )
,
, rowspan=2, 0+
, rowspan=2,
, rowspan=2,
, -
, β−n (< )
,
, -
, rowspan=2,
, rowspan=2 style="text-align:right" , 12
, rowspan=2 style="text-align:right" , 19
, rowspan=2,
, rowspan=2,
, β− ()
,
, rowspan=2, 1/2+
, rowspan=2,
, rowspan=2,
, -
, β−n ()
,
, -
, rowspan=2,
, rowspan=2 style="text-align:right" , 12
, rowspan=2 style="text-align:right" , 20
, rowspan=2,
, rowspan=2,
, β− ()
,
, rowspan=2, 0+
, rowspan=2,
, rowspan=2,
, -
, β−n ()
,
, -
, rowspan=3,
, rowspan=3 style="text-align:right" , 12
, rowspan=3 style="text-align:right" , 21
, rowspan=3,
, rowspan=3,
, β− ()
,
, rowspan=3, 3/2−
, rowspan=3,
, rowspan=3,
, -
, β−n ()
,
, -
, β−2n ?Decay mode shown is energetically allowed, but has not been experimentally observed to occur in this nuclide.
, ?
, -
, rowspan=3,
, rowspan=3 style="text-align:right" , 12
, rowspan=3 style="text-align:right" , 22
, rowspan=3,
, rowspan=3,
, β− (> )
,
, rowspan=3, 0+
, rowspan=3,
, rowspan=3,
, -
, β−n ()
,
, -
, β−2n (< )
,
, -
, rowspan=3,
, rowspan=3 style="text-align:right" , 12
, rowspan=3 style="text-align:right" , 23
, rowspan=3,
, rowspan=3,
, β−n ()
,
, rowspan=3, (3/2−, 5/2−)
, rowspan=3,
, rowspan=3,
, -
, β− ()
,
, -
, β−2n ?
, ?
, -
, rowspan=3,
, rowspan=3 style="text-align:right" , 12
, rowspan=3 style="text-align:right" , 24
, rowspan=3,
, rowspan=3,
, β− ()
,
, rowspan=3, 0+
, rowspan=3,
, rowspan=3,
, -
, β−n ()
,
, -
, β−2n ?
, ?
, -
, rowspan=3,
, rowspan=3 style="text-align:right" , 12
, rowspan=3 style="text-align:right" , 25
, rowspan=3,
, rowspan=3,
, β− ?
, ?
, rowspan=3, (3/2−)
, rowspan=3,
, rowspan=3,
, -
, β−n ?
, ?
, -
, β−2n ?
, ?
, -
, rowspan=3,
, rowspan=3 style="text-align:right" , 12
, rowspan=3 style="text-align:right" , 26
, rowspan=3, #
, rowspan=3, 2# ms , β− (100# %)
, #
, rowspan=3, 0+
, rowspan=3,
, rowspan=3,
, -
, β−n ?
, ?
, -
, β−2n ?
, ?
, -
, rowspan=2, ?This isotope has not yet been definitively observed; given data is inferred or estimated from periodic trends.
, rowspan=2 style="text-align:right" , 12
, rowspan=2 style="text-align:right" , 27
, rowspan=2, #
, rowspan=2, <
, n ?
, ?
, rowspan=2, 7/2−#
, rowspan=2,
, rowspan=2,
, -
, β− ?
, ?
, -
, rowspan=3,
, rowspan=3 style="text-align:right" , 12
, rowspan=3 style="text-align:right" , 28
, rowspan=3, #
, rowspan=3, 1# ms , β− ?
, ?
, rowspan=3, 0+
, rowspan=3,
, rowspan=3,
, -
, β−n ?
, ?
, -
, β−2n ?
, ?
, -
, rowspan=2, ?
, rowspan=2 style="text-align:right" , 12
, rowspan=2 style="text-align:right" , 29
, rowspan=2, #
, rowspan=2,
, β− ?
, ?
, rowspan=2, 3/2−#
, rowspan=2,
, rowspan=2,
, -
, β−n ?
, ?
External links
Magnesium isotopes data from ''The Berkeley Laboratory Isotopes Project's''
References
{{Navbox element isotopes MagnesiumMagnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ta ...