MCPA Use In The USA In 2017 on:
[Wikipedia]
[Google]
[Amazon]
MCPA (2-methyl-4-chlorophenoxyacetic acid) is a powerful, selective, widely used phenoxy herbicide. The pure compound is a brown-colored powder. MCPA has been extensively used in agriculture to control broad-leaf weeds as a growth regulator primarily in
 MCPA is used as an herbicide, generally as its salt or esterified forms. Used thus, it controls broadleaf weeds, including
MCPA is used as an herbicide, generally as its salt or esterified forms. Used thus, it controls broadleaf weeds, including
4-chloro-2-methylphenol)
is the intermediate in the synthesis of phenoxy herbicides, and is also the
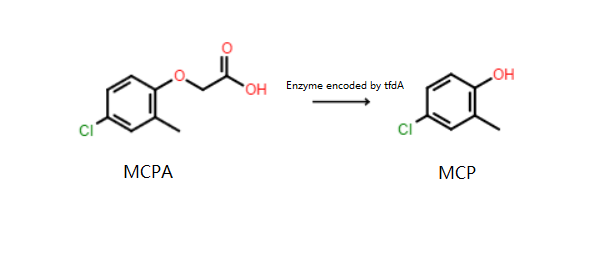 The MCPA can be degraded biologically in soils by plants and microorganisms. The major metabolite of MCPA degradation is MCP
The MCPA can be degraded biologically in soils by plants and microorganisms. The major metabolite of MCPA degradation is MCP
4-chloro-2-methylphenol)
The pathway could be the cleavage of the
(4-Chloro-2-hydroxymethylphenoxyacetic acid)
Recent studies have demonstrated that biological degradation of MCPA is enzymatically catalyzed by an α-ketoglutarate-dependent dioxygenase encoded by the tfdA gene of soil microorganisms. Soil indigenous bacteria that carry the tfdA gene could use MCPA as the sole source of carbon.
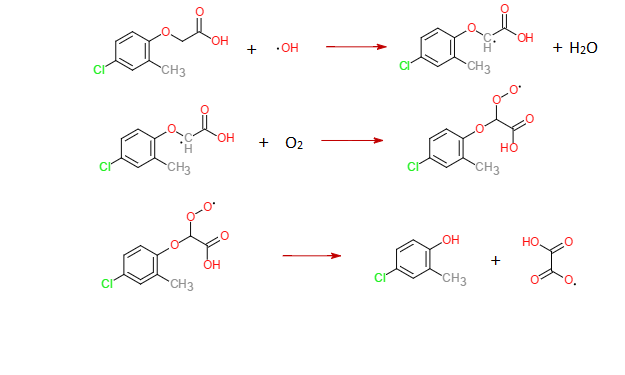
 MCPA also could be photochemically degraded. Two scheme pathways can be proposed for the formation of the main intermediate, MCP. One scheme is MCPA oxidation by hydroxyl radical, •OH. The hydroxyl radical adds on the ring, followed by radical transfer to the ether carbon. With oxygen present, the addition of the hydroxyl radical leads the cleavage of the ether link, yielding MCP. The other scheme is MCPA oxidation by positive
MCPA also could be photochemically degraded. Two scheme pathways can be proposed for the formation of the main intermediate, MCP. One scheme is MCPA oxidation by hydroxyl radical, •OH. The hydroxyl radical adds on the ring, followed by radical transfer to the ether carbon. With oxygen present, the addition of the hydroxyl radical leads the cleavage of the ether link, yielding MCP. The other scheme is MCPA oxidation by positive
4-chloro-2-methylphenylformate
With the presence of oxygen, the positive holes h+ oxidation finally yields MCP as well.
pasture
Pasture (from the Latin ''pastus'', past participle of ''pascere'', "to feed") is land used for grazing. Pasture lands in the narrow sense are enclosed tracts of farmland, grazed by domesticated livestock, such as horses, cattle, sheep, or swine ...
and cereal crops field since 1945. The mode of action of MCPA is as an auxin
Auxins (plural of auxin ) are a class of plant hormones (or plant-growth regulators) with some morphogen-like characteristics. Auxins play a cardinal role in coordination of many growth and behavioral processes in plant life cycles and are essenti ...
, which are growth hormones that naturally exist in plants. Overdose application of MCPA acts as an herbicide and results in abnormal growth.
History
In 1936 investigations began at ICIs Jealott's Hill research center into the effects ofauxin
Auxins (plural of auxin ) are a class of plant hormones (or plant-growth regulators) with some morphogen-like characteristics. Auxins play a cardinal role in coordination of many growth and behavioral processes in plant life cycles and are essenti ...
s on plant growth looking specifically for a way to kill weeds without harming crops such as wheat and oats. William Templeman found that when indole-3-acetic acid (IAA), the naturally occurring auxin, was used at high concentrations, it could stop plant growth. In 1940, he published his finding that IAA killed broadleaf plants within a cereal field.
Templeman and the ICI group were searching for compounds with similar or greater selective activity than IAA or 1-naphthaleneacetic acid
1-Naphthaleneacetic acid (NAA) is an organic compound with the formula C10H7CH2CO2H. This colorless solid is soluble in organic solvents. It features a carboxylmethyl group (CH2CO2H) linked to the "1-position" of naphthalene.
Use and regulation
N ...
in inhibiting the growth of weeds while not adversely affecting the growth of cereal
A cereal is any Poaceae, grass cultivated for the edible components of its grain (botanically, a type of fruit called a caryopsis), composed of the endosperm, Cereal germ, germ, and bran. Cereal Grain, grain crops are grown in greater quantit ...
crops. They synthesized MCPA from the corresponding phenol by exposing it to chloroacetic acid and dilute base in a straightforward substitution reaction:
:2-methyl-4-chlorophenol + ClCH2CO2H + base → MCPA + base·HCl (hydrochloric acid)
By the end of 1941 it was clear to the Templeman group that MCPA was one of the most active compounds tested but other auxin herbicides including 2,4-D were also effective. This work took place during World War II
World War II or the Second World War, often abbreviated as WWII or WW2, was a world war that lasted from 1939 to 1945. It involved the vast majority of the world's countries—including all of the great powers—forming two opposin ...
and was a case of multiple discovery. Four groups worked independently in the United Kingdom and the United States: the ICI team; Philip S. Nutman and associates at Rothamsted Research in the UK; Franklin D. Jones and associates at the American Chemical Paint Company; and Ezra Kraus, John W. Mitchell, and associates at the University of Chicago
The University of Chicago (UChicago, Chicago, U of C, or UChi) is a private research university in Chicago, Illinois. Its main campus is located in Chicago's Hyde Park neighborhood. The University of Chicago is consistently ranked among the b ...
and the United States Department of Agriculture
The United States Department of Agriculture (USDA) is the United States federal executive departments, federal executive department responsible for developing and executing federal laws related to farming, forestry, rural economic development, ...
. All four groups were subject to wartime secrecy laws and did not follow the usual procedures of publication and patent disclosure, although ICI did file an application relating to both MCPA and 2,4-D on 7 April 1941 in the UK. In December 1942, following a meeting at the Ministry of Agriculture the Rothamsted and ICI workers pooled resources and Nutman moved to Jealott's Hill to join the ICI effort. The first publications about this group of herbicides were by other workers who were not the original inventors: the precise sequence of discovery events has been discussed.
MCPA was first reported in the open scientific literature by Slade, Templeman and Sexton in 1945. ICI's decision to commercialize MCPA (rather than 2,4-D, for example) was influenced by the fact that ICI had access to 2-methyl-4-chlorophenol and following extensive field trials the material was first made available to UK farmers in 1946, as a 1% dust.
Mode of action
MCPA acts by mimicking the action of the plant growth hormoneauxin
Auxins (plural of auxin ) are a class of plant hormones (or plant-growth regulators) with some morphogen-like characteristics. Auxins play a cardinal role in coordination of many growth and behavioral processes in plant life cycles and are essenti ...
, which results in uncontrolled growth and eventually death in susceptible plants, mainly dicotyledon
The dicotyledons, also known as dicots (or, more rarely, dicotyls), are one of the two groups into which all the flowering plants (angiosperms) were formerly divided. The name refers to one of the typical characteristics of the group: namely, t ...
s. It is absorbed through the leaves and is translocated to the meristems of the plant. Uncontrolled, unsustainable growth ensues, causing stem curl-over, leaf withering, and eventual plant death.
Commercial use
 MCPA is used as an herbicide, generally as its salt or esterified forms. Used thus, it controls broadleaf weeds, including
MCPA is used as an herbicide, generally as its salt or esterified forms. Used thus, it controls broadleaf weeds, including thistle
Thistle is the common name of a group of flowering plants characterised by leaves with sharp prickles on the margins, mostly in the family Asteraceae. Prickles can also occur all over the planton the stem and on the flat parts of the leaves. ...
and dock
A dock (from Dutch language, Dutch ''dok'') is the area of water between or next to one or a group of human-made structures that are involved in the handling of boats or ships (usually on or near a shore) or such structures themselves. The ex ...
, in cereal crops and pasture
Pasture (from the Latin ''pastus'', past participle of ''pascere'', "to feed") is land used for grazing. Pasture lands in the narrow sense are enclosed tracts of farmland, grazed by domesticated livestock, such as horses, cattle, sheep, or swine ...
. It is selective for plants with broad leaves, and this includes most deciduous
In the fields of horticulture and Botany, the term ''deciduous'' () means "falling off at maturity" and "tending to fall off", in reference to trees and shrubs that seasonally shed leaves, usually in the autumn; to the shedding of petals, aft ...
trees. Clover
Clover or trefoil are common names for plants of the genus ''Trifolium'' (from Latin ''tres'' 'three' + ''folium'' 'leaf'), consisting of about 300 species of flowering plants in the legume or pea family Fabaceae originating in Europe. The genus ...
s are tolerant at moderate application levels. It is currently classified as a restricted use pesticide
Restricted use pesticides (RUP) are pesticides not available to the general public in the United States. Fulfilling its pesticide regulation responsibilities, the United States Environmental Protection Agency (EPA) registers all pesticides and ins ...
in the United States
The United States of America (U.S.A. or USA), commonly known as the United States (U.S. or US) or America, is a country primarily located in North America. It consists of 50 states, a federal district, five major unincorporated territorie ...
: its use is mapped by the US Geological Survey, whose data show consistent use from 1992, with a small recent decline in the ten years to 2017, the latest date for which figures are available. The compound is now used almost exclusively in wheat
Wheat is a grass widely cultivated for its seed, a cereal grain that is a worldwide staple food. The many species of wheat together make up the genus ''Triticum'' ; the most widely grown is common wheat (''T. aestivum''). The archaeologi ...
.
Its toxicity and biodegradation are topics of current research. One formulation is described by its manufacturer as "designed for specific markets that require the safest possible phenoxy product, primarily for use in the Pacific Northwest". Though not extremely toxic, it has been determined that MCPA can form complexes with metal ions and thereby increase their bioavailability, and there is also work being done to utilize this ability.
Chemical use
Because it is inexpensive, MCPA is used in various chemical applications. Itscarboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
group allows the formation of conjugated complexes with metals (see above). The acid functionality makes MCPA a versatile synthetic intermediate for more complex derivatives.
Brand names
The following commercial products contain MCPA: * Agritox, Agroxone, Chiptox, Chwastox, Cornox, Methoxone, Rhonox, Spurge Power, Tigrex, Verdone Extra (UK), Weed-Rhap, Weed'n'Feed, Weed-B-Gone, Zero Bindii & Clover Weeder (Aus), Jolt (Aus), BIN-DIE (Aus), Maatilan MCPA, K-MCPA, Hedonal, Basagran (Finland), and others.Degradation in soil
Since MCPA is extensively used in the USA, the extensively dispersed MCPA and its biological and photochemical metabolites might be deemable as environmentally hazardous. However, current studies show that there is no resistance of MCPA to degrade in soil.Behaviors in soil
MCPA herbicide is usually sprayed to the soil surface and plant leaves in its water solution, sometimes with additionalsurfactant
Surfactants are chemical compounds that decrease the surface tension between two liquids, between a gas and a liquid, or interfacial tension between a liquid and a solid. Surfactants may act as detergents, wetting agents, emulsifiers, foaming ...
. MCPA in soil can be absorbed by plant roots, and translocated in phloem to leaves and stems. The MCPA residue left in soil typically has a half-life of 24 days. However, the degradation rate depends on environmental conditions, such as temperature and soil moisture. MCPA is rather mobile in soil, and not strongly adsorbed to soil particles, with Kf = 0.94 and 1/n = 0.68 of Freundlich adsorption.
Environmental risks
Wide usage of MCPA as an herbicide raises concern of environmental risks, so considerable research has been done in recent decades to evaluate the environmental risk of MCPA. MCPA can be moderately toxic to mammal and aquatic organisms, and relatively less toxic to birds. MCP4-chloro-2-methylphenol)
is the intermediate in the synthesis of phenoxy herbicides, and is also the
metabolite
In biochemistry, a metabolite is an intermediate or end product of metabolism.
The term is usually used for small molecules. Metabolites have various functions, including fuel, structure, signaling, stimulatory and inhibitory effects on enzymes, c ...
of MCPA degradation. It has been estimated that a total of 15000 tons of MCP were produced in 1989 in the EU.UNEP publications, OECD SIDS, 4-chloro-2-methylphenol. http://www.inchem.org/documents/sids/sids/1570645.pdf MCP is considered very toxic to aquatic organisms. However, the concentration of MCPA and MCP detected in water and soil are lower than the predicted no-effect levels of all environmental compartments, and considered to present low potential risk.
The carboxyl group of MCPA can form conjugated complex with metals as a ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electr ...
. In the general pH range of aqueous environments, the MCPA-metal complex has higher solubility than metal ions. MCPA may be environmentally hazardous by affecting the mobility and bio-availability of heavy metals such as cadmium
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, silvery-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Like zinc, it demonstrates oxidation state +2 in most of ...
and lead
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cu ...
. The acid functionality makes MCPA a versatile synthetic intermediate for more complex derivatives
-COOH + M+ → -COOM + H+
Bio-degradation
 The MCPA can be degraded biologically in soils by plants and microorganisms. The major metabolite of MCPA degradation is MCP
The MCPA can be degraded biologically in soils by plants and microorganisms. The major metabolite of MCPA degradation is MCP4-chloro-2-methylphenol)
The pathway could be the cleavage of the
ether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be c ...
linkage, yielding MCP and acetate acid. Another pathway could be the hydroxylation of the methyl group, yielding cloxyfona(4-Chloro-2-hydroxymethylphenoxyacetic acid)
Recent studies have demonstrated that biological degradation of MCPA is enzymatically catalyzed by an α-ketoglutarate-dependent dioxygenase encoded by the tfdA gene of soil microorganisms. Soil indigenous bacteria that carry the tfdA gene could use MCPA as the sole source of carbon.
Photo-degradation

 MCPA also could be photochemically degraded. Two scheme pathways can be proposed for the formation of the main intermediate, MCP. One scheme is MCPA oxidation by hydroxyl radical, •OH. The hydroxyl radical adds on the ring, followed by radical transfer to the ether carbon. With oxygen present, the addition of the hydroxyl radical leads the cleavage of the ether link, yielding MCP. The other scheme is MCPA oxidation by positive
MCPA also could be photochemically degraded. Two scheme pathways can be proposed for the formation of the main intermediate, MCP. One scheme is MCPA oxidation by hydroxyl radical, •OH. The hydroxyl radical adds on the ring, followed by radical transfer to the ether carbon. With oxygen present, the addition of the hydroxyl radical leads the cleavage of the ether link, yielding MCP. The other scheme is MCPA oxidation by positive electron hole
In physics, chemistry, and electronic engineering, an electron hole (often simply called a hole) is a quasiparticle which is the lack of an electron at a position where one could exist in an atom or atomic lattice. Since in a normal atom or ...
s h+. The positive holes h+ polarize carboxyl group, CH2-COOH bond is split to produc4-chloro-2-methylphenylformate
With the presence of oxygen, the positive holes h+ oxidation finally yields MCP as well.
References
External links
* {{Herbicides Chloroarenes Acetic acids Auxinic herbicides Phenol ethers