This is a list of
cocaine
Cocaine (from , from , ultimately from Quechua: ''kúka'') is a central nervous system (CNS) stimulant mainly used recreationally for its euphoric effects. It is primarily obtained from the leaves of two Coca species native to South Am ...
analogues. A cocaine analogue is an (usually) artificial construct of a novel
chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
from (often the starting point of natural) cocaine's molecular structure, with the result product sufficiently similar to cocaine to display similarity in, but alteration to, its chemical function. Within the scope of analogous compounds created from the structure of cocaine, so named "cocaine analogues" retain 3''β''-benzoyloxy or similar functionality (the term specifically used usually distinguishes from
phenyltropane
Phenyltropanes (PTs) were originally developed to reduce cocaine addiction and dependency. In general these compounds act as inhibitors of the plasmalemmal monoamine reuptake transporters. Although RTI holds a strong position in this field, ...
s, but in the broad sense generally, as a category, includes them) on a tropane skeleton, as compared to other stimulants of the kind. Many of the semi-synthetic cocaine analogues ''proper'' which have been made & studied have consisted of among the nine following classes of compounds:
* stereoisomers of cocaine
* 3''β''-phenyl ring substituted analogues
* 2''β''-substituted analogues
* ''N''-modified analogues of cocaine
* 3''β''-carbamoyl analogues
* 3''β''-alkyl-3-benzyl tropanes
* 6/7-substituted cocaines
* 6-alkyl-3-benzyl tropanes
* piperidine homologues of cocaine
However strict analogues of cocaine would also include such other potential combinations as phenacyltropanes & other carbon branched replacements not listed above. The term may also be loosely used to refer to drugs manufactured from cocaine or having their basis as a
total synthesis
Total synthesis is the complete chemical synthesis of a complex molecule, often a natural product, from simple, commercially-available precursors. It usually refers to a process not involving the aid of biological processes, which distinguishes i ...
of cocaine, but modified to alter their effect &
QSAR. These include both intracellular sodium channel blocker anaesthetics and stimulant
dopamine reuptake inhibitor
A dopamine reuptake inhibitor (DRI) is a class of drug which acts as a reuptake inhibitor of the monoamine neurotransmitter dopamine by blocking the action of the dopamine transporter (DAT). Reuptake inhibition is achieved when extracellular ...
ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's elect ...
s (such as certain, namely tropane-bridged-excised,
piperidine
Piperidine is an organic compound with the molecular formula (CH2)5NH. This heterocyclic amine consists of a six-membered ring containing five methylene bridges (–CH2–) and one amine bridge (–NH–). It is a colorless liquid with an odor de ...
s). Additionally, researchers have supported combinatorial approaches for taking the most promising analogues currently elucidated and mixing them to the end of discovering novel & efficacious compounds to optimize their utilization for differing distinct specified purposes.
Analogs ''sensu stricto''
Cocaine Stereoisomers

There are eight
stereoisomers
In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms ...
of cocaine (excluding
mesomers and modifications to the internal portion of the tropane ring). Due to the presence of four asymmetric carbon atoms in the 1- & 5- to 8 (N) position bond bridge that could adopt ''R-'' & ''S-'' configurations, cocaine can be considered to have as many as sixteen stereoisomers. However, geometric constraints imparted by the bridgehead amine allow only eight to be created.
The natural isomerism of cocaine is unstable and prone to
epimer
In stereochemistry, an epimer is one of a pair of diastereomers. The two epimers have opposite configuration at only one stereogenic center out of at least two. All other stereogenic centers in the molecules are the same in each. Epimerization is ...
ization. For example, the end product of cocaine biosynthesis contains an axial C2-carbomethoxy moiety which readily undergoes
epimer
In stereochemistry, an epimer is one of a pair of diastereomers. The two epimers have opposite configuration at only one stereogenic center out of at least two. All other stereogenic centers in the molecules are the same in each. Epimerization is ...
ization to the equatorial position via
saponification
Saponification is a process of converting esters into soaps and alcohols by the action of aqueous alkali (for example, aqueous sodium hydroxide solutions). Soaps are salts of fatty acids, which in turn are carboxylic acids with long carbon chains. ...
.
For any 2D structural diagrams where stereochemistry is not indicated, it should be assumed the analogue depicted shares the stereochemical conformation of ''R''-cocaine unless noted otherwise.
Arene benzene-ring 2′, 3′, 4′ (5′ & 6′) position (
aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
) substitutions
''para''-substituted benzoylmethylecgonines
The
MAT binding pocket
In biology and biochemistry, the active site is the region of an enzyme where substrate molecules bind and undergo a chemical reaction. The active site consists of amino acid residues that form temporary bonds with the substrate (binding site) a ...
analogous to the lipophilic place on cocaine-like compounds, inclusive of the benzene ring, is approximate to 9
Å in length. Which is only slightly larger than a phenyl ring by itself.
''meta''-substituted benzoylmethylecgonines
*
ɑIC50 value for displacement of sup>3Hocaine
''ortho''-substituted benzoylmethylecgonines
*
ɑIC50 value for displacement of sup>3Hocaine
The hydroxylated 2′-OH analogue exhibited a tenfold increase in potency over cocaine.
Manifold and termination benzoyloxy phenyl-substitutions
Multi-substitutions (substitutions of substitutions; e.g.
''meta''- & ''para''-) or manifold ("many-fold") substituted analogues are analogues where more than one modification from the parent molecule takes place (having numerous intermediary constituents). These are created with often surprising structure–activity relationship results extrapolated therefrom. It is even a common case where two separate substitutions can each yield a weaker, lower affinity or even wholly non-efficacious compound respectively; but due to findings that oftentimes, when used together, such two mutually inferior changes being added in tandem to one analogue has the potential to make the resultant derivative display much greater efficacy, affinity, selectivity &/or strength than even the parent compound; which otherwise was compromised by either of those two alternations when made alone.
Benzoyl and carbomethoxy branch modifications
*
Benzoylthiomethylecgonine
Benzoylthiomethylecgonine is a cocaine analog which includes a sulfur in replacement of an oxygen on the single bonded benzoyl ester, resulting in lower electronegativity than the parent compound.
See also
*Salicylmethylecgonine
Salicylmeth ...

A sulfur in place of the oxygen at the benzoyl ester single bond results in a lower electronegativity than that of cocaine.
*
Cocaine reverse ester (REC)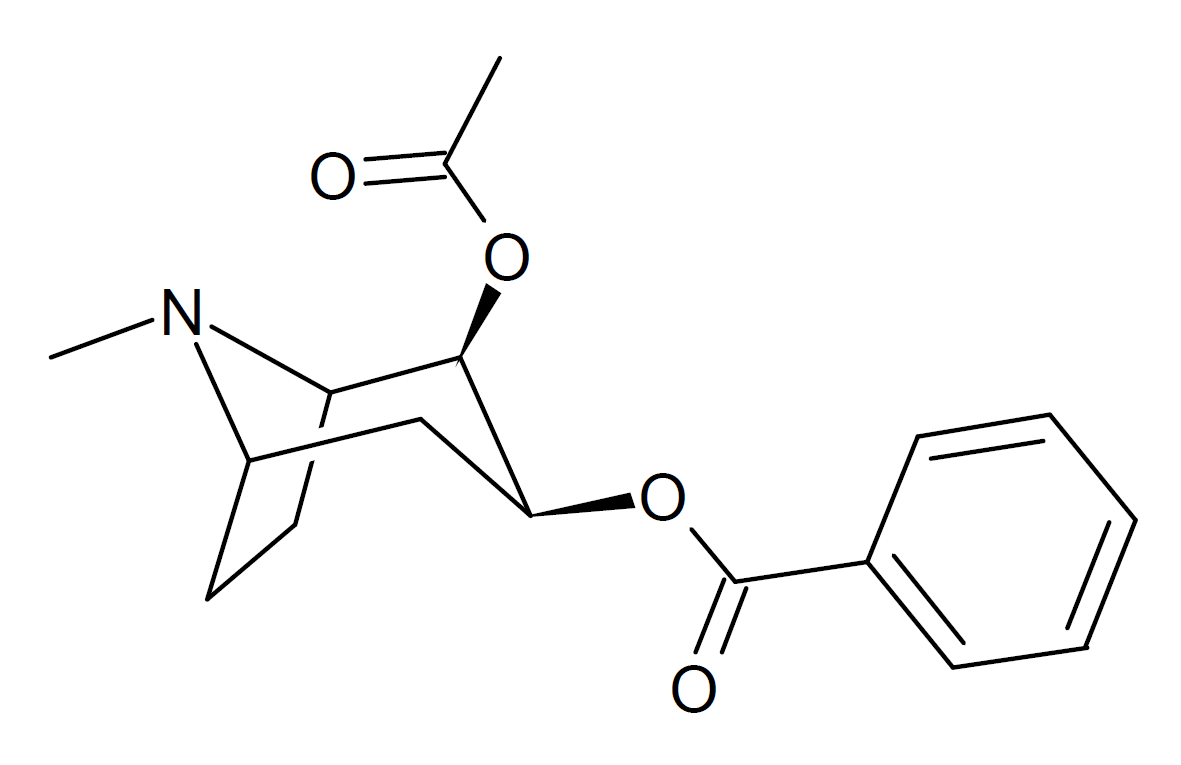
REC is a cocaine analogue which contains a "reversed" C2 carbomethoxy moiety. In animal studies, REC lacked cocaine-like stimulant effects.
C1-tropane-ring hydrogen—substitutions
*
ɑ, ''P'' < 0.05 compared with (—)-cocaine (one-way ANOVA followed by Dunnett's multiple comparisons test)
*
b, ''P'' < 0.01 compared with (—)-cocaine (one-way ANOVA followed by Dunnett's multiple comparisons test)
*
cLidocaine was found to have a value of 39.6 ± 2.4, the weakest of all tested.
*
dSame reference gives 25.9 ± 2.4 μM for (+)-cocaine and 13.6 ± 1.3 μM for norcocaine. Comparably it gives 12.7 ± 1.5 μM for the sigmaergic affinity of (+)-amphetamine. Another reference gives 1.7-6.7 μM for (—)-cocaine. All values ''K''i.
*
Using same data-set as above table, the following compounds were found to compare as:
**
CFT @ DAT = 39.2 ± 7.1 (n = 5)
**
fluoxetine @ SERT = 27.3 ± 9.2 (n = 3)
**
desipramine @ NET = 2.74 ± 0.59 (n = 3)
Cocaine analogs substituting the C1-tropane ring position, requiring sulfinimine (''N''-sulfinyl-imine) chemistry (before the innovation of which were untenable) which bind unlike the typical configuration at DAT (open to out) as cocaine (with its terminal D79-Y156 distance of 6.03 Å), or in the atypical (closed to out) conformation of the benztropines (3.29 Å). Though closer to the open to out: (—)-1-methyl-cocaine = 4.40 Å & (—)-1-phenyl-cocaine = 4.89 Å, and exhibiting preferential interaction with outward facing DAT conformation, they appear to have the lack of behavioral stimulation as-like the closed to out type. Despite having non-stimulant behavior profiles, they still seem to have anti-depressant behavioral profiles.
The C1 phenyl analog is ten times stronger than cocaine as a dopamine reuptake pump ligand, and twenty four times stronger as a local anesthetic (voltage-dependent Na+ channel blocker), whereas the C1 methyl analog is 2.3 times less potent as a local anesthetic.
''cf.''
hydroxytropacocaine for a natural alkaloid (lacking however, the 2-position carbmethoxy) that is a C1 substituent with a
hydroxy group
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
.
2''β''-substitutions
Compounds 196e-h possess greater SERT affinity than cocaine, but possess weaker NET/DAT affinities (with the exception of 196g at NET). Compounds 196k, 196n, 196o, and 197c all possess greater DAT affinity than cocaine. Compound 197b (dimethyl amide) displayed a 1,131-fold increased selectivity in affinity over the serotonin transporter, with only slight reductions in potency for the dopamine & norepinephrine transporters. Whereas 197c (
Weinreb amide
The Weinreb–Nahm ketone synthesis is a chemical reaction used in organic chemistry to make carbon–carbon bonds. It was discovered in 1981 by Steven M. Weinreb and Steven Nahm as a method to synthesize ketones. The original reaction involved ...
, N-methoxy-N-methyl amide) had a 469× increase at
SERT, with greater affinity for
DAT than cocaine and an equal
NET affinity. 197b was 137×, and 196c 27× less potent at binding to the serotonin transporter, but both had a NET / DAT ratio that made for a better
dopaminergic
Dopaminergic means "related to dopamine" (literally, "working on dopamine"), dopamine being a common neurotransmitter. Dopaminergic substances or actions increase dopamine-related activity in the brain. Dopaminergic brain pathways facilitate do ...
than cocaine. The consideration that large, bulky C2 substituents would alter the spatial conformation of the tropane ring system by distorting the piperidine portion of the system and thus hamper binding appears to be unfounded.
Benzoylecgonine (197e) is the inactive primary metabolite of cocaine generated through hydrolysis of the C2 methyl ester. ''In vitro'' binding studies indicate that benzoylecgonine is ~2,200x less potent than cocaine at the dopamine transporter, possibly due to
zwitterion formation
Formation may refer to:
Linguistics
* Back-formation, the process of creating a new lexeme by removing or affixes
* Word formation, the creation of a new word by adding affixes
Mathematics and science
* Cave formation or speleothem, a secondar ...
preventing strong DAT binding. In contrast to ''in vitro'' studies, the lack of activity observed in ''in vivo'' studies is likely the result of reduced
blood–brain barrier
The blood–brain barrier (BBB) is a highly selective semipermeable border of endothelial cells that prevents solutes in the circulating blood from ''non-selectively'' crossing into the extracellular fluid of the central nervous system where ne ...
penetration than formation of a
zwitterion.
Bioisostere 2-position carbmethoxy-ester functional replacements
Vinylogous 2''β''-position carbmethoxy-ester functional replacements
Compounds 201b & 201c were significantly more potent than cocaine while compounds 201a, 201d & 201e were significantly less potent. This finding indicates that the presence of a hydrogen bond acceptor (''i.e.'' carbomethoxy) at the 2''β'' position is not absolutely necessary for the creation of high affinity cocaine analogues.
''N''-modifications
*
ɑIC50 (nM) for displacement of sup>3HIN 35428
Tricyclic cocaine analogues
8 to 2 tethered analogues
''See
''N''-front & back bridged phenyltropanes.''
Back-bridged cocaine analogues are considered more akin to untethered cocaine analogs & phenyltropane derivatives (where the nitrogen
lone pair is not fixed or constrained) and better mimics their affinities. This is due to when the eighth carbon tropane position is freely rotatable and unbound it preferably occupies the ''axial'' position as defining its least energy & most unhindered state. In front-bridged analogs the nitrogen lone pairings rigid fixity makes it reside in an ''equatorial'' placing for the piperidine ring-part of the tropane nucleus, pointing to the two-carbon & three methylene unit bridgehead; giving the attested front-bridged cocaine analogues preference for SERT over DAT.
8 to 3 tethered analogues
*
"N/T" = "not tested"
Tropane ring contraction (azabornane) analogues
6/7 tropane position methoxycocaine & methoxypseudococaine analogues
3''β''-position 2′—(6′) & 2''β''-substitution combination analogues
*
ɑFor displacement of sup>3Haroxetine (5-HTT & NET)
*
bFor displacement of sup>3Hisoxetine (5-HTT & NET)
3''β''-Carbamoyl analogues
Phenyl 3-position linkage substitutions

See:
List of phenyltropanes (Many phenyltropanes are derived from cocaine metabolites, such as
methylecgonidine, as
precursors
Precursor or Precursors may refer to:
*Precursor (religion), a forerunner, predecessor
** The Precursor, John the Baptist
Science and technology
* Precursor (bird), a hypothesized genus of fossil birds that was composed of fossilized parts of unr ...
. Whereas fully synthetic methods have been devised from the starting material of vinylcarbenoids & pyrroles.)
The difference in the length of the benzoyloxy and the phenyl linkage contrasted between cocaine and phenyltropanes makes for a shorter distance between the
centroid
In mathematics and physics, the centroid, also known as geometric center or center of figure, of a plane figure or solid figure is the arithmetic mean position of all the points in the surface of the figure. The same definition extends to any ...
of the aromatic benzene and the bridge nitrogen of the tropane in the latter PTs. This distance being on a scale of 5.6
Å for phenyltropanes and 7.7
Å for cocaine or analogs with the benzoyloxy intact. This may account for PTs increased behavioral stimulation profile over cocaine. Differences in binding potency have also been explained considering solvation effects; cocaine containing 2''β'',3''β''-ester groups being calculated as more solvated than the WIN-type compounds (i.e. troparil). Higher p''K''
ɑs of the tropane nitrogen (8.65 for cocaine, 9.55 for troparil & 11.95 for vinyl analogue 43a), decreased aqueous solvation & decreased conformational flexibility added to increased binding affinity.




Despite the observation of increased stimulation, phenyltropanes lack the local anesthetic sodium channel blocking effect that the benzoyloxy imparts to cocaine. Beside topical affect, this gives cocaine an affinity for binding to sites on the dopamine and serotonin sodium dependent transport areas that are distinct & specific to MAT in contrast to the general sodium channels; creating a separate mechanism of relational affinity to the transporters in addition to its inhibition of the reuptake for those transporters; this is unique to the local anesthetic value in cocaine & analogues with a similar substitute for the benzoyloxy that leaves the sodium channel blockage ability intact. Rendering such compounds as different functionally in their relation to MAT contrasted to phenyltropane analogues which have the local anesthetic bridge removed. (Requiring some of the sodium ions to be pumped from the axon via
Na+/K+-ATPase). In addition, it even has been postulated that a crucial role regarding the electron energy imparted via voltage sensitization (and thus
action potential
An action potential occurs when the membrane potential of a specific cell location rapidly rises and falls. This depolarization then causes adjacent locations to similarly depolarize. Action potentials occur in several types of animal cells ...
blockage with a molecule capable of intersecting its specific channel, in the case of cocaine a
sodium channel
Sodium channels are integral membrane proteins that form ion channels, conducting sodium ions (Na+) through a cell's membrane. They belong to the superfamily of cation channels and can be classified according to the trigger that opens the chan ...
, that potentially serves in
re-quantifying its charge) upon a receptor binding site may attenuate the mediating influence of the inhibitory regulation that autoreceptors play by their slowing neurotransmitter release when an
efflux is created through an instance of agonism by a compound; allowing said efflux to be continued without the body's attempt to maintain
homeostasis
In biology, homeostasis ( British also homoeostasis) (/hɒmɪə(ʊ)ˈsteɪsɪs/) is the state of steady internal, physical, and chemical conditions maintained by living systems. This is the condition of optimal functioning for the organism and ...
enacting in as readily responsive a manner to its conformational change.
3''β''-Alkylphenyltropane & 3''β''-Alkenyl analogues
The compound 224e, the 3''β''-styrene analogue, had the highest potency in its group. While 224b & 224c showed the most selectivity, with 224b having a ten-fold greater potency for the dopamine transporter than cocaine.
6-Alkyl-3-benzyltropane analogues
''
N.B.'' The benzylidene derivatives serve as synthetic intermediates for 6-Alkyl-3-benzyltropanes and have not been assayed for biological activity. Compounds ''237a'' and ''238a'' are the same compound as both are the parent for either series with a hydrogen saturated in their respective substitution place.
Direct 2,3-pyrimidino fused
''cf.'' strobamine (at right) for a more efficacious compound as like the below.
*
"NA" = "no affinity", ''e.g.'' unquantifiable.
Direct di-hetero-benzene (pyrimidino) 2,3-fused and thus rigidified cocaine analogs.
Piperidine cocaine-homologues
''cf.'' phenyltropane piperidine-homologues for compounds with a more optimized conformation that yield higher affinities when binding to MAT.
Cocaine hapten analogues
*
ɑ6-(2R,3S)-3-(benzoyloxy)-8-methyl-8-azabicyclo .2.1/nowiki> octane-2-carbonyloxy-hexanoic acid
*
b6-(2R,3S)-3-(benzoyloxy)-8-methyl-8-azabicyclo .2.1/nowiki> octane-2-carboxamido-hexanoic acid

Cocaine haptens that create catalytic anti-bodies require transitional states as affected ''in vivo''. Monoclonal antibodies generated against
BSA-coupled 402e accelerated the rate of cocaine hydrolysis by ~23,000x and eliminated the reinforcing effects of cocaine administration in rats.
Structural/Functional intermediate analogues
Piperidine Analogues
*
JZ-IV-10
JZ-IV-10 is a piperidine derivative related to cocaine which acts as a highly potent serotonin–norepinephrine–dopamine reuptake inhibitor
A serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI), also known as a triple reuptak ...
(a "
Modafinil
Modafinil, sold under the brand name Provigil among others, is a central nervous system (CNS) stimulant medication used to treat sleepiness due to narcolepsy, shift work sleep disorder, and obstructive sleep apnea. While it has seen off-la ...
hybrid" with nocaine.
''cf.''
List of modafinil analogues)

*
Nocaine
(+)-CPCA (nocaine, 3α-carbomethoxy-4β-(4-chlorophenyl)-''N''-methylpiperidine aka CTDP 31,446
) is a stimulant drug similar in structure to pethidine (an opioid that possesses NDRI actions) and to RTI-31, but nocaine is lacking the two-carbo ...

A somewhat recent occurrence among tentative modern folklore which has traversed the circling of rumors mostly confined to the likes of universities and popular culture trivia has been that cocaine is one element, or molecule increment of weight or charge etc., away from the molecular structure of sugar. Though such a statement is false as a general pretense, there is a dextrose based super-structure that has a vaguely similar overlay with cocaine which is "benzoyl-''beta''-
D-glucoside."
*
Benzoyl-''beta''-D-glucoside

Benztropine (3α-Diphenylmethoxy Tropane) Analogues
ɑInhibition at 10 μM
The binding of benztropine analogues to the
DAT differs significantly from that of cocaine and the phenyltropanes. Benztropines are considered to be "atypical" DAT ligands because they stabilize the DAT in an inward-facing (closed-to-out) conformation, whereas cocaine and the phenyltropanes stabilize the DAT in an outward-facing (open-to-out) conformation. This difference in DAT binding may be responsible for the lack of cocaine-like behavioral effects observed in animal and human studies of the benztropine analogues and other “atypical” DAT inhibitors.
Studies of the structure-activity relationships of benztropine have shown that DAT affinity and selectivity over other monoamine transporters is enhanced by 4′,4′-difluorination. Modification of the tropane n-substituent was found to mitigate the anticholinergic effects of benztropine analogues by reducing
M1 affinity.
Tropanyl Isoxazoline Analogues
Compound 7a (''3′-methoxy-8-methyl-spiro(8-azabicyclo(3.2.1)octane-3,5′(4′H)-isoxazole'') allosterically enhances SERT binding of other reuptake ligands. Compound 7a construed as a potentiating allosteric effect (by unveiling occluded configured serotonin uptake-area ligand-site on surface of transporter that allows for binding by exogenous ligand, when SERT is otherwise conformed in a transitional manner where a SERT ligand cannot bind, this effect with compound in question occurs) at concentrations of 10μM—30μM (wherein it acts by interconverting the conformational state of unexposed SERTs to ones exposing the SSRI binding site via a shift to the equilibrium of the MAT) while exerting an inhibitory orthosteric effect when concentrations reach >30μM and above.
7a is the only known compound to allosterically modulate SERT in such a way within ''in vitro'' conditions (
tianeptine
Tianeptine, sold under the brand names Stablon and Coaxil among others, is an atypical antidepressant which is used mainly in the treatment of major depressive disorder, although it may also be used to treat anxiety, asthma, and irritable bowel ...
has been shown to do similar, but has only shown efficacy doing so in living ''in vivo'' tissue samples). Considering its noncompetitive inhibition of 5-HT transporters decreasing ''V''
max with small change in the ''K''
m for serotonin, putatively stabilizing the cytoplasm-facing conformation of SERT: in such respect it is considered to have the opposite effect profile of the anti-addiction drug
ibogaine
Ibogaine is a naturally occurring psychoactive substance found in plants in the family Apocynaceae such as '' Tabernanthe iboga'', '' Voacanga africana'', and '' Tabernaemontana undulata''. It is a psychedelic with dissociative properties.
Pre ...
(save for the function by which its anti-addictive properties are thought to be mediated, ''i.e.'' α
3β
4 nicotinic channel blockage. ''cf.''
18-Methoxycornaridine for such nicotinergic activity without the likewise SERT affinity).
Compound 11a possesses similar effects, but acts on the DAT. Similarly, such peripheral DAT considerations (when, as often is, considered conformational rather than otherwise explained as being electrostatic) may constitute the difference in affinity, through allosertic occulsion, between
cyclopentyl-ruthenium phenyltropane in its difference from the tricarbonyl-chromium
Alicyclic Amine Analogues
Dihydroimidazoles

See:
List of Mazindol analogues
Mazindol is usually considered a non-habituating (in humans, and some other mammals, but is habituating for ''e.g.''
Beagle
The beagle is a breed of small scent hound, similar in appearance to the much larger foxhound. The beagle was developed primarily for hunting hare, known as beagling. Possessing a great sense of smell and superior tracking instincts, th ...
s)
tetracyclic
Tetracyclics are cyclic chemical compounds that contain four interconnected rings of atoms, e.g. Tröger's base.
They have various pharmaceutical
A medication (also called medicament, medicine, pharmaceutical drug, medicinal drug or s ...
dopamine reuptake inhibitor (of somewhat spurious classification in the former).
It is a loosely functional analog used in cocaine research; due in large part to
''N''-Ethylmaleimide being able to inhibit approximately 95% of the specific binding of
sup>3Hazindol to the residues of the MAT binding site(s), however said effect of 10
mM ''N''-Ethylmaleimide was prevented in its entirety by just 10 ''μ''M cocaine. Whereas neither 300 ''μ''M dopamine or
D-amphetamine afforded sufficient protection to contrast the efficacy of cocaine.
Local anesthetics (not usually CNS stimulants)
In animal studies, certain of the local anesthetics have displayed residual
dopamine reuptake inhibitor
A dopamine reuptake inhibitor (DRI) is a class of drug which acts as a reuptake inhibitor of the monoamine neurotransmitter dopamine by blocking the action of the dopamine transporter (DAT). Reuptake inhibition is achieved when extracellular ...
properties,
although not normally ones that are easily available. These are expected to be more cardiotoxic than phenyltropanes. For example, dimethocaine has behavioral stimulant effects (and therefore not here listed below) if a dose of it is taken that is 10 times the amount of cocaine. Dimethocaine is equipotent to cocaine in terms of its anesthetic equivalency.
[ Intralipid "rescue" has been shown to reverse the cardiotoxic effects of sodium channel blockers and presumably those effects when from cocaine administered intravenously as well.
]
See also
 * Coca alkaloids, the ones relating to cocaine biosynthesis include: benzoylecgonine, ecgonidine, ecgonine, hydroxytropacocaine, methylecgonine cinnamate, tropacocaine & truxilline
* Cocaine metabolites (Human), which include: benzoylecgonine (BE), ecgonine methyl ester (EME), ecgonine, norcocaine, ''p''-hydroxycocaine, ''m''-hydroxycocaine, ''p''-hydroxybenzoylecgonine (''p''OHBE) & ''m''-hydroxybenzoylecgonine
*
* Coca alkaloids, the ones relating to cocaine biosynthesis include: benzoylecgonine, ecgonidine, ecgonine, hydroxytropacocaine, methylecgonine cinnamate, tropacocaine & truxilline
* Cocaine metabolites (Human), which include: benzoylecgonine (BE), ecgonine methyl ester (EME), ecgonine, norcocaine, ''p''-hydroxycocaine, ''m''-hydroxycocaine, ''p''-hydroxybenzoylecgonine (''p''OHBE) & ''m''-hydroxybenzoylecgonine
* Dopaminergic
Dopaminergic means "related to dopamine" (literally, "working on dopamine"), dopamine being a common neurotransmitter. Dopaminergic substances or actions increase dopamine-related activity in the brain. Dopaminergic brain pathways facilitate do ...
s
* Federal Analog Act
The Federal Analogue Act, , is a section of the United States Controlled Substances Act passed in 1986 which allows any chemical "substantially similar" to a controlled substance listed in Schedule I or II to be treated as if it were listed in ...
* Pharmacophore
* Pharmacopoeia
A pharmacopoeia, pharmacopeia, or pharmacopoea (from the obsolete typography ''pharmacopœia'', meaning "drug-making"), in its modern technical sense, is a book containing directions for the identification of compound medicines, and published by ...
* Pharmacokinetics
Pharmacokinetics (from Ancient Greek ''pharmakon'' "drug" and ''kinetikos'' "moving, putting in motion"; see chemical kinetics), sometimes abbreviated as PK, is a branch of pharmacology dedicated to determining the fate of substances administered ...
* Pharmacodynamics
Pharmacodynamics (PD) is the study of the biochemical and physiologic effects of drugs (especially pharmaceutical drugs). The effects can include those manifested within animals (including humans), microorganisms, or combinations of organisms ...
Common analogues to prototypical D-RAs
Ras or RAS may refer to:
Arts and media
* RAS Records Real Authentic Sound, a reggae record label
* Rundfunk Anstalt Südtirol, a south Tyrolese public broadcasting service
* Rás 1, an Icelandic radio station
* Rás 2, an Icelandic radio sta ...
:
:*Substituted amphetamine
Substituted amphetamines are a class of compounds based upon the amphetamine structure; it includes all derivative compounds which are formed by replacing, or substituting, one or more hydrogen atoms in the amphetamine core structure with s ...
s
:* Substituted cathinones
:*Substituted phenethylamine
Substituted phenethylamines (or simply phenethylamines) are a chemical class of organic compounds that are based upon the phenethylamine structure; the class is composed of all the derivative compounds of phenethylamine which can be formed by ...
s
:*Substituted phenylmorpholine
Substituted phenylmorpholines, or substituted phenmetrazines alternatively, are chemical derivatives of phenylmorpholine or of the psychostimulant drug phenmetrazine. Most such compounds act as releasers of monoamine neurotransmitters, and have s ...
s
:*Substituted methylenedioxyphenethylamine
Substitution reaction, Substituted methylenedioxy- phenethylamines (MDxx) are a large chemical class of derivative (chemistry), derivatives of the phenethylamines, which includes many psychoactive drugs that act as entactogens, psychedelic drug, ...
s
Notes (inclu. specific locations of citations from within references used)
References
External links
U. S. Provisional Patent Application listing examples of compounds which are tropanes for prospective use in research
and their CAS Registry Number
A CAS Registry Number (also referred to as CAS RN or informally CAS Number) is a unique identification number assigned by the Chemical Abstracts Service (CAS), US to every chemical substance described in the open scientific literature. It inclu ...
s
{{DEFAULTSORT:Cocaine Analogues
Cocaine
Local anesthetics
Euphoriants
Carboxylate esters
Methyl esters
Tropanes
Chemical classes of psychoactive drugs


 See: List of phenyltropanes (Many phenyltropanes are derived from cocaine metabolites, such as methylecgonidine, as
See: List of phenyltropanes (Many phenyltropanes are derived from cocaine metabolites, such as methylecgonidine, as  *
*